Abstract
Objective
Materials and Methods
Results
Conclusion
Notes
This article had been presented at "2014 Annual Meeting of Society of Korean Endovascular Neurosurgeon" under the title of "Rebleeding of ruptured intracranial aneurysms at immediate postoperative period after coil embolization". We certify that the contents of this manuscript, in all or in part, has not been published and is not being considered for publication elsewhere, unless otherwise specified herein.
References
Fig. 1
A case with discrepancies in the aneurysm size and morphology between computed tomography angiography (CTA) and three-dimensional rotational angiography (3DRA) findings. (A) Brain CT shows a large amount of subarachnoid hemorrhage (SAH) in the basal cistern, interhemispheric fissure, and both Sylvian fissures. (B) CTA shows an elongated anterior communicating artery aneurysm with a small neck diameter of 1.44 mm and a large neck-to-dome diameter of 3.57 mm. The dome-to-neck ratio of the aneurysm is 2.47. (C, D) Digital subtraction angiography and 3DRA performed after CTA show a small saccular aneurysm with a dome-to-neck ratio of 1.83 (neck diameter, 1.22 mm; neck-to-dome diameter, 2.24 mm). (E) The aneurysm is secured by coil embolization using 3 detachable coils, with no neck and lumen remnants. (F) Compared to immediate postoperative brain CT, brain CT performed 8 h after coil embolization shows increased SAH in the left Sylvian fissure, interhemispheric fissure, and basal cistern with newly developed intraventricular hemorrhage.

Fig. 2
A case of rebleeding of anterior communicating artery (ACoA) aneurysms after successful coil embolization. (A) Brain computed tomography (CT) of a 58-year-old male shows dense subarachnoid hemorrhage. (B) Computed tomography angiography (CTA) shows a saccular aneurysm of the ACoA (arrow). Cerebral digital subtraction angiography (DSA) and three-dimensional rotational angiography (3DRA) show 2 ACoA aneurysms. (C, D) A large aneurysm in the superior direction (arrowhead), and (E, F) a small aneurysm in the anterior direction (arrowhead). (G, H) Both aneurysms are successfully obliterated with detachable coils, and there is no definite evidence of neck and lumen remnants. Because a coil loop protrudes into the ACoA (arrow), continued anticoagulation therapy with intravenous low-molecular-weight heparin is performed. After the sudden presentation of comatose mentality, brain CT shows increased subarachnoid hemorrhage (SAH) in the basal cistern. (I) Contrast leakage from the ACoA aneurysm in the anterior direction is confirmed on follow-up DSA (arrowhead). (J) Additional coil embolization secures the reruptured aneurysm.
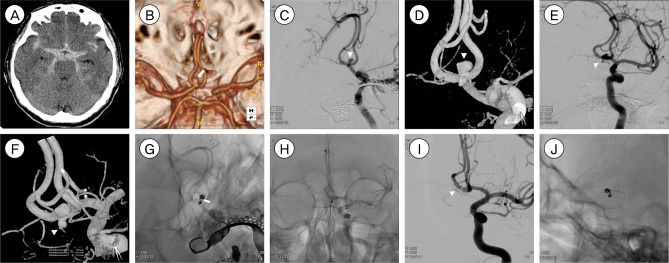
Table 1
Clinical and angiographic characteristics of patients with rebleeding of ruptured intracranial aneurysms after coil embolization
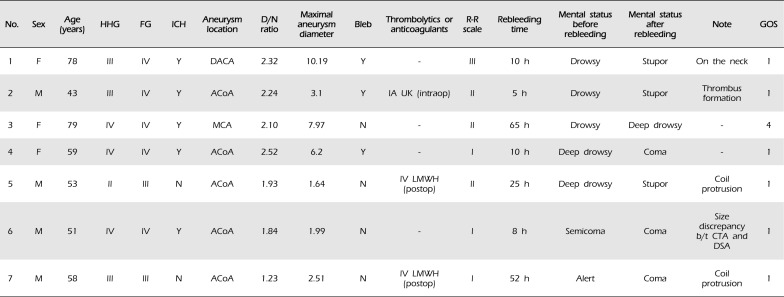
HHG = Hunt-Hess grade; FG = Fisher grading system; ICH = intracerebral hemorrhage; D/N = dome/neck; R-R = Raymond-Roy; GOS = Glasgow Outcome Scale; F = female; M = male; Y = yes; N = No; DACA = distal anterior cerebral artery aneurysm; ACoA = anterior communicating artery; MCA = middle cerebral artery; IA = intraarterial; IV = intravenous; UK = urokinase; intraop = intraoperative; LMWH = low-molecular-weight heparin; postop = postoperative; b/t = between; CTA = computed tomography angiography; DSA = digital subtraction angiography
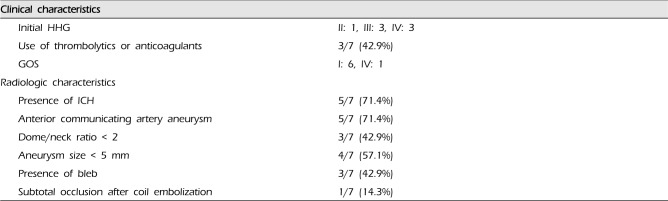
Table 2
Summary of the characteristics of patients with rebleeding of ruptured intracranial aneurysms after coil embolization




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download