This article has been
cited by other articles in ScienceCentral.
Abstract
Objective
Terson's syndrome, a complication of visual function, has occasionally been reported in patients with aneurysmal subarachnoid hemorrhage (SAH), however the factors responsible for Terson's syndrome in aneurysmal SAH patients have not yet been fully clarified. In this study, we report on potential risk factors for prediction and diagnosis of Terson's syndrome in the earlier stage of the disease course in patients with aneurysmal SAH.
Materials and Methods
The authors retrospectively analyzed the data of 322 consecutive patients who suffered from aneurysmal SAH in a single institution between Jan. 2007 and Dec. 2013. Medical records including demographics, neurologic examination, and radiologic images were collected to clarify the risk factors of Terson's syndrome. Patients with visual problem were consulted to the Department of Ophthalmology.
Results
Among 332 patients with aneurysmal SAH, 34 patients were diagnosed as Terson's syndrome. Four individual factors, including World Federation of Neurosurgical Societies (WFNS) grade at admission, aneurysm size, method of operation, and Glasgow outcome scale showed statistically significant association with occurrence of Terson's syndrome. Of these, WFNS grade at admission, aneurysm size, and method of operation showed strong association with Terson's syndrome in multivariate analysis. Terson's syndrome accompanied by papilledema due to increased intracranial pressure led to permanent visual complication.
Conclusion
In patients with aneurysmal SAH, the patients' WFNS grade at admission, the size of the aneurysms, particularly the diameter of the aneurysm dome, and the method of operation might influence development of Terson's syndrome.
Go to :

Keywords: Aneurysm, Subarachnoid hemorrhage, Vitreous hemorrhage, Incidence, Risk factors
INTRODUCTION
Terson's syndrome has been defined as an intraocular hemorrhage in the vitreous region. However, the definition of Terson's syndrome as vitreous hemorrhage alone has been expanded owing to the close connection among the pathogenesis of retinal, subhyaloid, and vitreous hemorrhage. According to previous studies, Terson's syndrome has been reported in approximately 8-44% of such cases.
1)8)10)11)
However, the incidence of Terson's syndrome might be underestimated due to the severity of aneurysmal subarachnoid hemorrhage (SAH) and patients' grave systemic condition. Accordingly, the incidence of Terson's syndrome might have been much higher if previous studies had reported the predictive and risk factors of Terson's syndrome. Therefore, the aim of this study is to evaluate risk factors of Terson's syndrome for earlier diagnosis of aneurysmal SAH patients and to administer prompt treatment in the proper situation. If the treatment is delayed, epiretinal membrane and macular hole followed by Terson's syndrome could result in worsening of visual acuity and visual field.
Go to :

MATERIALS AND METHODS
Patient population
A retrospective study was conducted to determine the predictive and risk factors of Terson's syndrome. Using our medical center's database, 332 consecutive patients admitted due to aneurysmal SAH between Jan. 2007 and Dec. 2013 were selected, and patients' medical records and radiologic data were attained. The SAH was confirmed using computed tomography (CT) in all cases. After the diagnosis, brain CT angiography or digital subtraction angiography (DSA) was performed to determine the location of the ruptured cerebral aneurysms. The patients selectively underwent surgery by aneurysmal neck clipping or endovascular coil embolization at the senior author's discretion. Two patients with comorbid ophthalmologic disease, one with diabetic optic neuropathy, and one with retinal detachment, as well as 452 patients with traumatic SAH and 23 patients with non-aneurysmal SAH were excluded. This study was approved by the institutional review board of the author's institute (HYIRB IRB No. 2014-07-016-002).
Image study and outcome assessment
Patients' initial mental status and World Federation of Neurosurgical Societies (WFNS) grade were examined by subgroup analysis for comparison of the outcome of Terson's syndrome. Fisher's grade was determined by brain CT scan prior to the operation, and brain CT angiography or DSA provided detailed information about the aneurysm; rupture location and size, maximum diameter from the dome to the neck of the aneurysm. Initial systolic blood pressure on visit and patients' history of hypertension and smoking were also included in order to find correlative factors in occurrence of Terson's syndrome. Patients were assessed using the Glasgow outcome scale (GOS) 6 months after the operation.
Ophthalmic examination
Patients' with visual complaints, such as blurred vision, discomfort of eye movement, and diplopia were all requested for evaluation to the Ophthalmology Department. For evaluation of Terson's syndrome, fundoscopy was performed as an initial screening test in every case in the perioperative period, and, subsequent ultrasonography and fluorescein angiography were performed for definite diagnosis. Only fundoscopic examination was performed as a screening tool for patients who patient showed unstable vital signs or were admitted directly to the intensive care unit.
As pointed out previously, patients with retinal, subhyaloid, and vitreous hemorrhage were diagnosed as Terson's syndrome. Pars plana vitrectomy was performed on lesions requiring surgical treatment, while conservative treatment was administered in others. Patients with Terson's syndrome were subsequently checked by the Ophthalmology Department, and after discharge, serial follow-up was done at the outpatient clinic.
Statistical analysis
Statistical analysis of the data was performed using the Fisher's exact test or the Mann-Whitney U test for the univariate analysis. In multivariate analysis, the individual factors showing a p value of < 0.20 in the univariate analysis were included. These meaningful factors were held into the logistic regression model to control for the confounding factors and a p value of < 0.05 was regarded as a statistically significant result. All statistics were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
Go to :

RESULTS
Characteristics of the study population
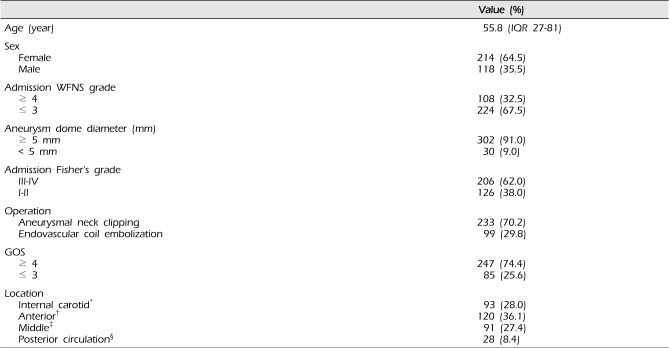
A total of 332 consecutive patients were diagnosed as aneurysmal SAH between Jan. 2007 and Dec. 2013. Of these, 118 patients were male and 214 were female, ranging in age from 27 to 84 with a median of 55. In our study population of 322 patients, microsurgical neck clipping (73.8%) was performed in 245 patients, much more frequently than endovascular coil embolization. WFNS grade over 4 was found in 108 patients, and, 206 patients showed higher Fisher's grade (grade III, IV) in the whole study group (62.0%). The demographic and clinical data of 322 consecutive patients with aneurysmal SAH are shown in
Table 1.
Table 1
Characteristics of 332 consecutive patients with aneurysmal subarachnoid hemorrhage
|
Value (%) |
|
Age (year) |
55.8 (IQR 27-81) |
|
Sex |
|
|
Female |
214 (64.5) |
|
Male |
118 (35.5) |
|
Admission WFNS grade |
|
|
≥ 4 |
108 (32.5) |
|
≤ 3 |
224 (67.5) |
|
Aneurysm dome diameter (mm) |
|
|
≥ 5 mm |
302 (91.0) |
|
< 5 mm |
30 (9.0) |
|
Admission Fisher's grad |
|
|
III-IV |
206 (62.0) |
|
I-II |
126 (38.0) |
|
Operation |
|
|
Aneurysmal neck clipping |
233 (70.2) |
|
Endovascular coil embolization |
99 (29.8) |
|
GOS |
|
|
≥ 4 |
247 (74.4) |
|
≤ 3 |
85 (25.6) |
|
Location |
|
|
Internal carotid*
|
93 (28.0) |
|
Anterior†
|
120 (36.1) |
|
Middle‡
|
91 (27.4) |
|
Posterior circulation§
|
28 (8.4) |

The size of the neck to the dome of aneurysms over 5 mm was found in 302 of 332 patients (91.0%). The most frequent rupture location of the aneurysm was the anterior cerebral artery (ACA) territory (36.1%) and the posterior circulation showed the lowest frequency (8.4%).
Six months after aneurysmal neck clipping or endovascular coil embolization, three quarters of the patients showed good recovery, with a score of GOS 4 or 5.
Factors associated with Terson's syndrome
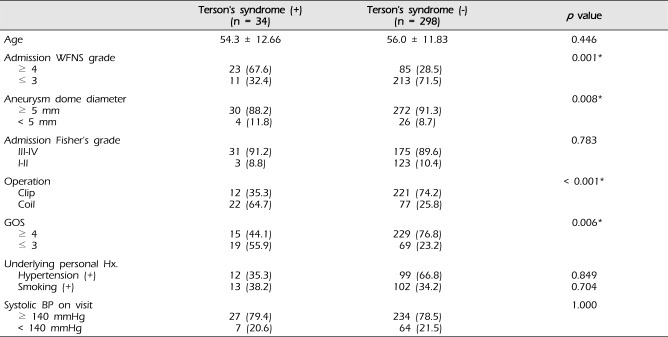
Among the 332 aneurysmal SAH patients, 34 patients were diagnosed as Terson's syndrome. Univariate analysis was performed in two groups between patients diagnosed as Terson's syndrome and patients without Terson's syndrome. The patients' age was similar between two groups (54.3 ± 12.66 vs. 56.0 ± 11.83). Fisher's grade at admission, systolic blood pressure on visit, previous history of hypertension, and smoking showed no statistically significant differences between the two groups (
Table 2). However, four individual factors, including WFNS grade at admission, aneurysm dome diameter, method of operation, and GOS showed statistically significant association with development of Terson's syndrome (
p < 0.01).
Table 2
Comparison of variable factors affecting occurrence of Terson's syndrome in patients with aneurysmal subarachnoid hemorrhage (n = 332)
|
Terson's syndrome (+)
(n = 34) |
Terson's syndrome (-)
(n = 298) |
p value |
|
Age |
54.3 ± 12.66 |
56.0 ± 11.83 |
0.446 |
|
Admission WFNS grade |
|
|
0.001*
|
|
≥ 4 |
23 (67.6) |
85 (28.5) |
|
≤ 3 |
11 (32.4) |
213 (71.5) |
|
Aneurysm dome diameter |
|
|
0.008* |
|
≥ 5 mm |
30 (88.2) |
272 (91.3) |
|
< 5 mm |
4 (11.8) |
26 (8.7) |
|
Admission Fisher's grade |
|
|
0.783 |
|
III-IV |
31 (91.2) |
175 (89.6) |
|
I-II |
3 (8.8) |
123 (10.4) |
|
Operation |
|
|
< 0.001*
|
|
Clip |
12 (35.3) |
221 (74.2) |
|
Coil |
22 (64.7) |
77 (25.8) |
|
GOS |
|
|
0.006*
|
|
≥ 4 |
15 (44.1) |
229 (76.8) |
|
≤ 3 |
19 (55.9) |
69 (23.2) |
|
Underlying personal Hx. |
|
|
|
|
Hypertension (+) |
12 (35.3) |
99 (66.8) |
0.849 |
|
Smoking (+) |
13 (38.2) |
102 (34.2) |
0.704 |
|
Systolic BP on visit |
|
|
1.000 |
|
≥ 140 mmHg |
27 (79.4) |
234 (78.5) |
|
< 140 mmHg |
7 (20.6) |
64 (21.5) |

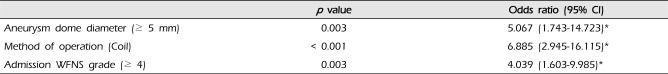
Meaningful independent risk factors of Terson's syndrome were assessed by logistic regression analysis. Three factors showed statistically significant correlation in development of Terson's syndrome: WFNS grade at admission (odds ratio [OR] 4.039, 95% confidence interval [CI] 1.603-9.985;
p < 0.01), aneurysm dome diameter (OR 5.067, 95% CI 1.743-14.723;
p < 0.01), and method of operation (endovascular coil embolization) (OR 6.885, 95% CI 2.945-16.115;
p < 0.01) (
Table 3).
Table 3
Independent risk factors of Terson's syndrome in patients with aneurysmal subarachnoid hemorrhage
|
p value |
Odds ratio (95% CI) |
|
Aneurysm dome diameter (≥ 5 mm) |
0.003 |
5.067 (1.743-14.723)*
|
|
Method of operation (Coil) |
< 0.001 |
6.885 (2.945-16.115)*
|
|
Admission WFNS grade (≥ 4) |
0.003 |
4.039 (1.603-9.985)*
|

Go to :

DISCUSSION
Evaluation of Terson's syndrome
Terson's syndrome has been reported in 8-44% of patients with spontaneous SAH.
1)8)10)11) This wide variation was caused by the obscured definition of Terson's syndrome and the retrospective nature of the clinical studies by different categorized groups.
1)8)10)11) Traditional Terson's syndrome has been defined as SAH patients combined with vitreous hemorrhage. However, within the groups of SAH, the clinical consensus statement of Terson's syndrome has shifted from not only the vitreous hemorrhage but also to include the subhyaloid and retinal hemorrhage groups. This is because the mechanisms in development of these ocular hemorrhages interact.
2)7)13) In addition, in previous retrospective studies, not all patients were screened by ophthalmologic examination. For diagnosis of Terson's syndrome, ophthalmologic screening with fundoscopy must be performed within the first few hours because of a sudden increase of intracranial pressure due to cerebral edema, hydrocephalus, and associated intracerebral hemorrhage.
5) Within 12 days after the aneurysm rupture, serial screening should be performed for evaluation of the true incidence of Terson's syndrome even in subclinical patients with intraocular hemorrhages.
6)10) Regarding earlier screening test, Swallow et al.
12) reported that a CT scan can be helpful in diagnosis of Terson's syndrome in approximately 66.7% of patients. However in the case of indefinite CT scan images in the area of the retina or vitreous body, ultrasonography could be used in confirming Terson's syndrome or other ophthalmologic problems in the follow up hospital course.
4)10)
Risk factors for Terson's syndrome
Despite its high incidence, factors related to Terson's syndrome have rarely been described in previous studies.
3)7) In the current study, we focused on clarifying risk factors of Terson's syndrome to ensure a better prognosis and to provide the basic foundation for future prospective studies.
Similar to a few available studies, in this study, intraocular hemorrhage showed significant association with the initial WFNS grade, GOS, and the size of the aneurysms.
3)7) In aneurysmal SAH patients, initial WFNS grade over 4 can be a risk factor for ongoing development of Terson's syndrome (
p < 0.01). This can be explained by the increased intracranial pressure, which can act as a trigger point in the onset of Terson's syndrome.
Aneurysm size exceeding 5mm, which has a higher rupture risk in patients with unruptured aneurysms compared with size under 5mm, showed a statistically significant association Terson's syndrome (
p < 0.01). This may be due to increase in the size of the aneurysm, thereby enhancing the hemodynamic stress of the cerebral vessel walls.
9) Sequentially, at the time of the rupture, the intracranial pressure would also be elevated along with the increased size of the long axis of the aneurysm. However, due to the relatively high proportion of Fisher's grade III and IV (62.0%) patients in the current study, we were unable to determine the correlation between Terson's syndrome and the amount of hemorrhage.
Fountas et al.
1) mentioned that location of an aneurysm in the anterior circulation, particularly anterior communicating aneurysm might influence development of Terson's syndrome. We also conjectured that aneurysms arising from the anterior circulation could be a more frequent cause of Terson's syndrome. The highest incidence of Terson's syndrome from aneurysms in the internal carotid territory might be associated with the highest increase of intracranial pressure among the anterior circulation aneurysms, as discussed in the study by Wang et al.,
14) whereas, aneurysmal SAH from the posterior circulation only accounts for 8.7% of the whole study group, and among them, only 2 cases have been diagnosed as Terson's syndrome. Regarding the method of operation involved, currently, aneurysms of the posterior circulation are usually treated by endovascular coil embolization. The hypothesis is that during microsurgical aneurysmal neck clipping, release of cerebrospinal fluid (CSF) might prevent the occurrence of hydrocephalus in the acute stage of aneurysmal SAH. Thus, unless the patients underwent extraventricular drainage, endovascular coil embolization techniques are likely to result in increased intracranial pressure.
5)13)
Because our research was conducted retrospectively, there may be selection bias, but also individual factors favoring decisions regarding surgery in aneurysmal SAH patients can affect the outcome. In addition, due to the relatively small portion of patients with Terson's syndrome, these statistically significant risk factors might have been overestimated. Thus, further evaluation by a large group study is required.
Go to :

CONCLUSION
The prevalence rate of Terson's syndrome as an ophthalmologic complication is relatively high in aneurysmal SAH patients. In the current study, several independent factors including aneurysm dome diameter, WFNS grade at admission, and method of operation such as endovascular intervention were associated with Terson's syndrome. Basic ophthalmologic examination (fundoscopy) should be performed as early as possible in all patients with aneurysmal SAH, particularly when the aneurysm size is over 5 mm and/or WFNS grade is over 4. In addition, if the patient underwent endovascular coil embolization, further ophthalmologic evaluation should be performed irrespective of aneurysm location as long as conditions permit. In the future, lest we should omit missing patients due to death and subclinical symptoms, more studies prospective studies are required in order to obtain more accurate information about Terson's syndrome.
Go to :








 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download