Abstract
Objective
Routine use of prophylactic antiepileptic drugs (AED) has been debated. We retrospectively evaluated the effects of prophylactic AED on clinical outcomes in patients with a good clinical grade suffering from aneurysmal subarachnoid hemorrhage (aSAH).
Materials and Methods
Between September 2012 and December 2014, 84 patients who met the following criteria were included: (1) presence of a ruptured aneurysm; (2) Hunt-Hess grade 1, 2, or 3; and (3) without seizure presentation. Patients were divided into two groups; the AED group (n = 44) and the no AED group (n = 40). Clinical data and outcomes were compared between the two groups.
Results
Prophylactic AEDs were used more frequently in patients who underwent microsurgery (84.1%) compared to those who underwent endovascular surgery (15.9%, p < 0.001). Regardless of prophylactic AED use, seizure episodes were not observed during the six-month follow-up period. No statistical difference in clinical outcomes at discharge (p = 0.607) and after six months of follow-up (p = 0.178) were between the two groups. After six months, however, favorable outcomes in the no AED group tended to increase and poor outcomes tended to decrease.
Conclusion
No difference in the clinical outcomes and systemic complications at discharge and after six months of follow-up was observed between the two groups. However, favorable outcomes in the no AED group showed a slight increase after six months. These findings suggest that discontinuation of the current practice of using prophylactic AED might be recommended in patients with a good clinical grade.
Administration of antiepileptic drugs (AED) in the setting of aneurysmal subarachnoid hemorrhage (aSAH) has become routine in the hope of achieving better outcomes for patients. Previous studies have reported a high incidence of seizures in the setting of aSAH (12.5-22%).4)23) AED use is standard practice for prevention of seizure episodes, which can cause hypoxic brain injury or subsequent electrical field suppression, possibly delaying recovery or adversely influencing the natural course of the disease. However, prophylactic AEDs have been associated with side effects including behavioral and cognitive impairments,1)16) and complicated brain hemorrhage. Therefore, more sophisticated guidelines for treatment or prevention of seizure due to SAH are needed.
According to the current guidelines,8)22) prophylactic AED may be considered for patients with known risk factors for seizure, such as poor clinical grade (Hunt-Hess [HH] grade 4 or 5), intraparenchymal hematoma, microsurgical clipping, postoperative hematoma, aneurysms of the middle cerebral artery, and cerebral ischemia.5)11)13)14)19) Among these risk factors, the most comprehensive and predictive entity is poor clinical grade such as in cases of middle cerebral arterial aneurysm with intraparenchymal hematoma requiring microsurgical clipping combined with decompressive surgery. However, the use of prophylactic AED in patients with a good clinical grade is not recommended by the guidelines but used currently in our clinical practice. Thus, we retrospectively evaluated the effects of prophylactic AED on clinical outcomes in patients with a good clinical grade suffering from aSAH.
This retrospective study was approved by our institutional review board, and the requirement for informed consent was waived. A total of 84 patients were enrolled from a database of 133 patients with aSAH who underwent either microsurgery or endovascular surgery between September 2012 and December 2014. All included patients met the following criteria: (1) presence of a ruptured intracranial aneurysm on computed tomography angiography or magnetic resonance angiography; (2) HH grade 1, 2, or 3 on admission; and (3) without seizure presentation. The remaining 49 patients from the database were excluded for the following reasons: (1) onset seizures (n = 4); (2) HH grade 4 or 5 on admission (n = 38); (3) history of previous seizure taking AED (n = 2); and (4) loss to follow-up (n = 5). Included patients were divided into two groups according to the usage of prophylactic AED; the AED group (n = 44) and the no AED group (n = 40). Patients were prescribed 1000 mg of levetiracetam or 900 mg valproic acid daily for at least 6 months (6 to 12 months).
Clinical data, including age, gender, initial HH grade, Fisher grade, hypertension, diabetes, dyslipidemia, smoking, treatment modality, hydrocephalus, external ventricular drainage, shunt, symptomatic vasospasm, procedure related complications, systemic complications and aneurysm characteristics (location, shape, size, and neck diameter), were reviewed and compared between the two groups. Procedure related complications were defined as new neurological deterioration related to treatment modality (microsurgery or endovascular surgery) or complications requiring additional procedures. Systemic complications were defined as major medical complications, such as infectious conditions (pneumonia or urinary tract infection), hepatic dysfunction, renal dysfunction, pulmonary complications (atelectasis, pulmonary effusion, or pulmonary edema), cardiology complications (myocardial infarction, arrhythmia, or heart failure), and electrolyte imbalance (hyponatremia or hypernatremia), affecting clinical outcomes. The patients' clinical outcomes were evaluated at discharge and after six months of follow-up using the modified Rankin scale (mRS). A favorable outcome was defined as mRS of 0 to 2 and a poor outcome as mRS of 3 to 6. No difference in overall medical management and general care in the acute period was observed between the two groups.
All statistical analyses were consulted to a biostatistician and performed using SPSS 22.0 (SPSS Inc., Chicago, IL, USA). Variables are expressed as the mean ± SD, or the number of patients (%), as appropriate. Student's t-tests were used for numeric variables, and Fisher's exact tests for nominal variables. Logistic regression analysis was performed on variables with an unadjusted effect and a p-value < 0.10 in univariate analysis to determine risk factors associated with poor outcomes in the AED group. A p-value < 0.05 for a 95% confidence interval (CI) was considered statistically significant.
The clinical characteristics and outcomes of the 84 patients according to the use of prophylactic AED are summarized in Table 1. The mean age was 52.6 ± 13.3 years (range: 25-86 years). Age, gender, underlying disease, and aneurysm profiles were not statistically different between the two groups. There was no occurrence of seizure episodes in any of the included patients during admission or follow-up. Treatment modality differed significantly between the two groups; prophylactic AED was used more frequently in patients who underwent microsurgery (84.1%) compared to those who underwent endovascular surgery (15.9%, p < 0.001). Aneurysm shape was also found to differ between the two groups (p = 0.032) because all dissecting aneurysms were treated by endovascular surgery.
A total of nine systemic complications occurred with higher frequency (20.5%) in the AED group compared to the no AED group (5.0%). However, statistical significance was not achieved (p = 0.106). The frequency of each complication is shown in Table 2. Pneumonia, in four patients, was the most common systemic complication. No identifiable psychiatric problem, delirium, or hepatic dysfunction was observed even in patients treated with AED. Regardless of prophylactic AED use, there was no occurrence of seizure episodes in any patients (HH grade 1, 2, or 3) with a SAH during six months of follow-up.
No statistical difference in clinical outcomes at discharge and after six months of follow-up was observed between the two groups (p = 0.607 and 0.178, respectively). At discharge, 38 (86.4%) of 44 patients in the AED group showed a favorable outcome with one death due to pneumonia sepsis whereas 36 (90.0%) of 40 patients in the no AED group showed favorable outcomes without mortality (Fig. 1). After six months of follow-up, patients in the AED group did not show improved clinical outcomes. However, favorable outcomes of patients in the no AED group showed a slight increase to 95.0% and poor outcomes decreased to 5.0%.
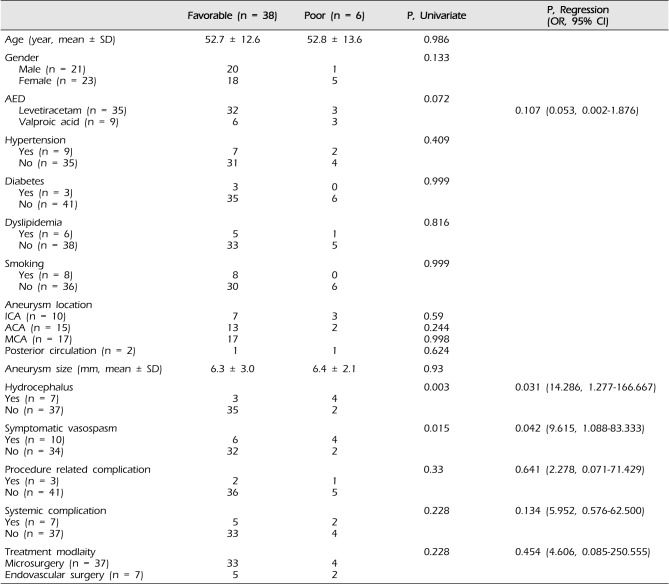
Analysis of risk factors associated with poor outcomes in the AED group is shown in Table 3. Logistic regression analysis showed that hydrocephalus (odds ratio [OR] = 14.286; 95% CI, 1.277 to 166.67; p = 0.031) and symptomatic vasospasm (OR = 9.615, 95% CI, 1.088 to 83.333; p = 0.042) were independently associated with poor outcomes in the AED group.
In the current study, seizure did not occur in aSAH patients with a good clinical grade during admission or follow-up regardless of prophylactic AED use. At our institution, we tended to use prophylactic AED for patients who underwent microsurgery and patients with ruptured aneurysms of the anterior cerebral artery. However, our findings indicate that treatment with AED had no effect on seizure prophylaxis. A number of retrospective series similar to ours have demonstrated no significant difference in terms of seizure outcome between groups with or without prophylaxis.3)6)9)15)18) In an analysis of four large trials, patients who received AED had an odds ratio of 1.56 for worse outcomes after three months of follow-up, as well as increased risk for vasospasm, neurological deterioration, cerebral infarction, and elevated temperature during hospitalization.18) In the current study, the clinical outcomes at discharge and after six months of follow-up were not statistically different between the two groups (p = 0.607 and 0.178, respectively). At discharge, 86.4% of patients in the AED group showed a favorable outcome, but there was one death due to pneumonia. In the no AED group, 90.0% of patients showed favorable outcomes without mortality. However, after six months of follow-up, favorable outcomes of patients in the no AED group showed a slight increase to 95.0% and poor outcomes decreased to 5.0%.
In clinical practice, AED usage for patients with aSAH is generally accepted for prevention of seizure episodes. According to the current guidelines,8)22) however, prophylactic AED may be considered for patients with known risk factors for seizure, one of which is poor clinical grade (HH grade 4 or 5) on initial presentation. Unlike aSAH with a poor clinical grade, patients with a good clinical grade (HH grade 1, 2, or 3) had relatively lower risk of developing seizure due to aneurysm rupture. In addition, routine use of AED in those patients has recently come under question.21) As reported in a systematic review, the rate of early postoperative seizure was 2.3% and the rate of late postoperative seizure was 5.5%.20) The average time to late seizure was 7.45 months. Patients who experienced a late seizure were more likely to undergo repair with microsurgery than endovascular surgery. They concluded that routine perioperative AED usage did not seem to prevent seizures after aSAH. However, it should be noted that seizures might occur years after aSAH and careful monitoring for late complications remains important.
In the current study, we realized that we tended to use prophylactic AED for patients who underwent microsurgery. In the International Subarachnoid Aneurysm trial (ISAT), patients treated with microsurgery had a significantly higher risk of late seizure than those treated with endovascular surgery.17) In a survey conducted by the American Association of Neurological Surgeons, 24% of neurosurgeons surveyed routinely prescribed AED for three months after aSAH regardless of whether seizures occurred at presentation, in the hospital, or not at all.2)6)10)12) However, in a systematic review there was no significant difference in the incidence of early and late seizure between patients treated with AED (3.0% and 5.9%, respectively) and those not treated with AED (2.2% and 6.3%, respectively, p > 0.99).20)
From the risk factor analysis for poor outcomes in the AED group, hydrocephalus and symptomatic vasospasm were found to be were well-known and understandable risk factors. Although the adverse effects of AEDs have not been well characterized in patients with aSAH, adverse effects due to phenytoin were reported in 21.4% of patients.20) We used levetriacetam or valproic acid, and fortunately did not experience adverse effects.
There are several limitations to this study. First, it was a retrospective study based on our prospectively collected database with a small number of enrolled patients. Therefore the data do not represent the true incidence of late seizures. Second, we did not evaluate electroencephalograms (EEG), thus we could not determine the occurrence of non-convulsive seizures.
A previous study indicated that the incidence of non-convulsive seizure from intracranial hemorrhage is approximately 38%.7) However, as we enrolled only conscious patients with a good clinical grade, non-convulsive seizures would likely have been detected clinically. Third, clinical outcomes of both groups might be affected by treatment modality, which determined AED administration in our practice. This imbalance in frequency of treatment modalities should have influenced clinical outcomes. Finally, cognitive function was not evaluated. The use of anticonvulsants could decrease intelligence. In patients with aSAH, however, evaluation of cognitive function might be incomplete, time-consuming, and lack specificity. Accordingly, we only measured clinical outcomes with the mRS.
No difference in clinical outcomes and systemic complications at discharge and after six months of follow-up was observed between the two groups. However, favorable outcomes in the no AED group showed a slight increase after six months. Our findings suggest that discontinuation of the current practice of prophylactic AED use might be recommended in patients with a good clinical grade.
References
1. American Academy of Pediatrics. Behavioral and cognitive effects of anticonvulsant therapy. Committee on Drugs. Pediatrics. 1985; 10. 76(4):644–647. PMID: 3931047.
2. Baker CJ, Prestigiacomo CJ, Solomon RA. Short-term Perioperative Anticonvulsant Prophylaxis for the Surgical Treatment of Low-risk Patients with Intracranial Aneurysms. Neurosurgery. 1995; 11. 37(5):863–870. discussion 870-1PMID: 8559333.

3. Bidziński J, Marchel A, Sherif A. Risk of epilepsy after aneurysm operations. Acta neurochir (Wien). 1992; 3. 119(1-4):49–52. PMID: 1481752.

4. Cabral RJ, King TT, Scott DF. Epilepsy after two different neurosurgical approaches to the treatment of ruptured intracranial aneurysm. J Neurol Neurosurg Psychiatry. 1976; 11. 39(11):1052–1056. PMID: 1011015.

5. Chang IB, Cho BM, Shin DI, Shim YB, Park SH, Oh SM. Risk of seizures after operative treatment of ruptured cerebral aneurysms. J Korean Neurosurg Soc. 2001; 6. 30(6):705–710.
6. Claassen J, Peery S, Kreiter KT, Hirsch LJ, Du EY, Connolly ES, et al. Predictors and clinical impact of epilepsy after subarachnoid hemorrhage. Neurology. 2003; 1. 60(2):208–214. PMID: 12552032.

7. Claassen J, Perotte A, Albers D, Kleinberg S, Schmidt JM, Tu B, et al. Nonconvulsive seizures after subarachnoid hemorrhage: Multimodal detection and outcomes. Ann Neurol. 2013; 7. 74(1):53–64. PMID: 23813945.

8. Connolly ES, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. 2012; 6. 43(6):1711–1737. PMID: 22556195.
9. Fabinyi GC, Artiola-Fortuny L. Epilepsy after craniotomy for intracranial aneurysm. Lancet. 1980; 6. 1(8181):1299–1300. PMID: 6104098.

10. Gilmore E, Choi HA, Hirsch LJ, Claassen J. Seizures and CNS Hemorrhage: Spontaneous Intracerebral and Aneurysmal Subarachnoid Hemorrhage. Neurologist. 2010; 5. 16(3):165–175. PMID: 20445426.
11. Hart Y, Sneade M, Birks J, Rischmiller J, Kerr R, Molyneux A. Epilepsy after subarachnoid hemorrhage: the frequency of seizures after clip occlusion or coil embolization of a ruptured cerebral aneurysm: results from the International Subarachnoid Aneurysm Trial. J Neurosurg. 2011; 12. 115(6):1159–1168. PMID: 21819189.
12. Hasan D, Schonck RS, Avezaat CJ, Tanghe HL, van Gijn J, van der Lugt PJ. Epileptic seizures after subarachnoid hemorrhage. Ann Neurol. 1993; 3. 33(3):286–291. PMID: 8498812.
13. Hoh BL, Nathoo S, Chi YY, Mocco J, Barker FG 2nd. Incidence of seizures or epilepsy after clipping or coiling of ruptured and unruptured cerebral aneurysms in the nationwide inpatient sample database: 2002-2007. Neurosurgery. 2011; 9. 69(3):644–650. discussion 650PMID: 21499155.

14. Kim TY, Huh SK, Lee JW, Lee KC. Risk factors of seizures associated with the management of ruptured cerebral aneurysms. Korean J Cerebrovasc Surg. 2006; 1. 8(1):10–14.
15. Lin YJ, Chang WN, Chang HW, Ho JT, Lee TC, Wang HC, et al. Risk factors and outcome of seizures after spontaneous aneurysmal subarachnoid hemorrhage. Eur J Neurol. 2008; 5. 15(5):451–457. PMID: 18325027.

16. Meador KJ, Loring DW, Abney OL, Allen ME, Moore EE, Zamrini EY, et al. Effects of carbamazepine and phenytoin on EEG and memory in healthy adults. Epilepsia. 1993; Jan-Feb. 34(1):153–157. PMID: 8422849.

17. Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005; 9. 366(9488):809–817. PMID: 16139655.

18. Ogden JA, Utley T, Mee EW. Neurological and psychosocial outcome 4 to 7 years after subarachnoid hemorrhage. Neurosurgery. 1997; 7. 41(1):25–34. PMID: 9218292.

19. Olafsson E, Gudmundsson G, Hauser WA. Risk of epilepsy in long-term survivors of surgery for aneurysmal subarachnoid hemorrhage: a population-based study in Iceland. Epilepsia. 2000; 9. 41(9):1201–1205. PMID: 10999560.

20. Raper DM, Starke RM, Komotar RJ, Allan R, Connolly ES Jr. Seizures After Aneurysmal Subarachnoid Hemorrhage: A Systematic Review of Outcomes. World Neurosurgery. 2013; May-Jun. 79(5-6):682–690. PMID: 23022642.

21. Riordan KC, Wingerchuk DM, Wellik KE, Zimmerman RS, Sirven JI, Noe KH, et al. Anticonvulsant drug therapy after aneurysmal subarachnoid hemorrhage: a critically appraised topic. Neurologist. 2010; 11. 16(6):397–399. PMID: 21150393.
22. Steiner T, Juvela S, Unterberg A, Jung C, Forsting M, Rinkel G. European Stroke Organization. European Stroke Organization Guidelines for the Management of Intracranial Aneurysms and Subarachnoid Haemorrhage. Cerebrovasc Dis. 2013; 2. 35(2):93–112. PMID: 23406828.

23. Walton JN. The electroencephalographic sequelae of spontaneous subarachnoid haemorrhage. Electroencephalogr Clin Neurophysiol. 1953; 2. 5(1):41–52. PMID: 13033807.

Fig. 1
Bar graph of the modified Rankin scale (mRS) showing clinical outcomes of the two groups at discharge. AED = antiepileptic drug.
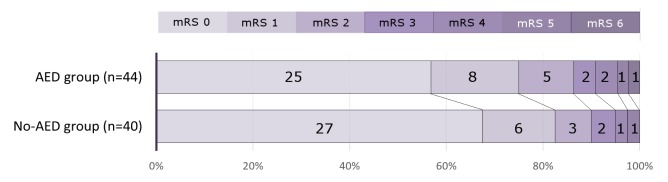
Table 1
Clinical data and outcomes of the two groups
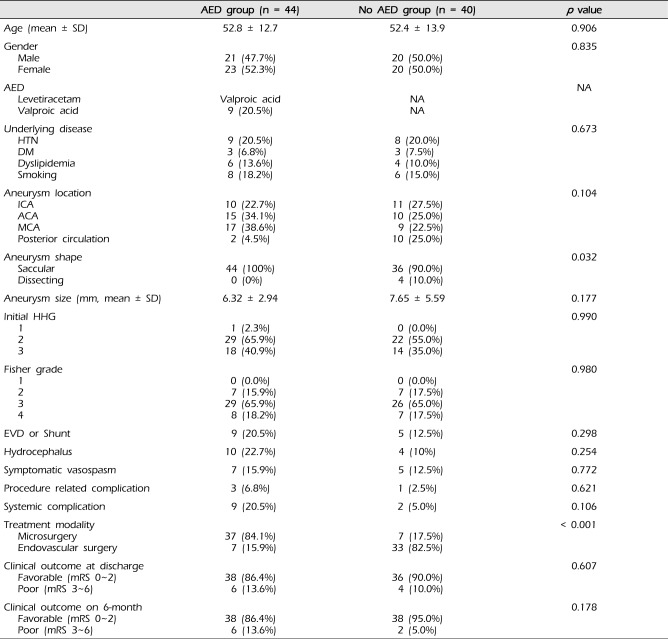
Table 2
Systemic complications in both groups
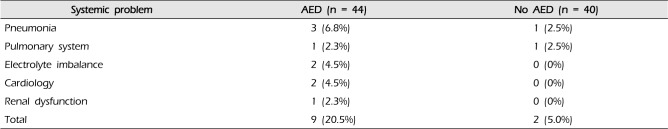
Table 3
Risk factors associated with poor outcomes in the AED group




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download