This article has been
cited by other articles in ScienceCentral.
Abstract
Objective
The objective of this study was to find out the clinical variables correlated with repeated intra-arterial (IA) nimodipine infusions in patients with medically refractory cerebral vasospasm (CV) following subarachnoid hemorrhage (SAH).
Materials and Methods
During the 36 months between January 2011 and December 2013, 275 patients were treated at our institute for SAH due to a ruptured intracranial aneurysm. Of the 275 patients, 26 patients (9.5%) met the inclusion criteria. For each patient, a retrospective review of their medical records was conducted.
Results
Eleven patients underwent a single IA nimodipine infusion and 15 patients underwent more than two IA nimodipine infusions. Multiple IA nimodipine infusion patients had poor improvement (2 of 15 patients, 13.3%) in Glasgow coma scale (GCS) scores after the first IA nimodipine infusion compared to patients of single IA nimodipine infusion (6 of 11 patients, 54.6%) (p = 0.038). The mean middle cerebral artery (MCA) Lindegaard ratio of multiple IA nimodipine infusion patients was 4.3 ± 1.1 after the first IA nimodipine infusion (p = 0.039). In multiple IA nimodipine infusion patients, CV occurred more often bilaterally (p = 0.035) and distally (p = 0.001). More vessel segments were affected in multiple IA nimodipine infusion patients (3.1 ± 1.0) (p < 0.001).
Conclusion
The following factors correlated with multiple IA nimodipine infusions: 1) no improvement in GCS after the IA nimodipine infusion; 2) no decrease of MCA velocity on transcranial doppler over 50 cm/s or Lindegaard ratio over 4.3 after the IA nimodipine infusion; 3) distal, bilateral, or diffuse involvement of CV.
Keywords: Cerebral vasospasm, Intra-Arterial, Nimodipine, Infusion
INTRODUCTION
Endovascular treatment for medically refractory cerebral vasospasm (CV) after subarachnoid hemorrhage (SAH) from aneurysm rupture has greatly improved over the past few decades. The most frequently used techniques are mechanical balloon angioplasty and intra-arterial (IA) administration of vasodilators. Although mechanical balloon angioplasty has a relatively long-lasting effects on narrowed vessels, the application of mechanical balloon angioplasty is limited to proximal vessels. Therefore, peripherally located diffuse CV and small arteries must be treated with vasodilators. However, the beneficial effects of vasodilators are short lived, and repeat treatment sessions are often necessary.
2)11) Among many vasodilating agents, IA nimodipine has been proven as effective and safe for the treatment of symptomatic CV after SAH.
2)3)4)6)7)8) IA nimodipine also has the advantages of acting on smaller distal vessels and diffuse vasospasm. However, its beneficial effects are temporary because of its short half-life. CV recurrence and incomplete treatment are the main issues with IA nimodipine treatment, and repeated IA infusions are often necessary.
IA infusion should be repeated or continued until a patient recovers fully from the critical situation of vasospasm. However, there is insufficient data on who benefits from repeated IA nimodipine infusions among patients with medically refractory CV. Our goal was to find out the clinical variables that correlate with repeated IA nimodipine infusion in patients with medically refractory CV following SAH.
MATERIALS AND METHODS
Patient population
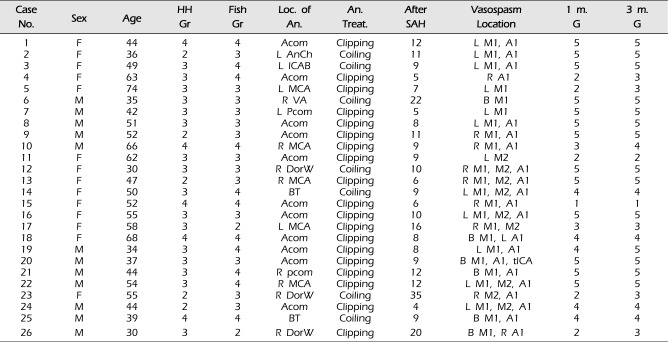
During the 36 months between January 2011 and December 2013, 275 patients were treated at our institute for SAH due to a ruptured intracranial aneurysm. Among the 275 patients, 26 patients (9.5%) had findings compatible with symptomatic CV. IA nimodipine infusions were used as routine endovascular treatment for symptomatic CV in these patients. Their medical records were reviewed retrospectively. In all patients, aneurysm treatment was performed within the first 24 hours after hemorrhage. All standard medical treatments for CV prevention were performed in the neurosurgical intensive care unit, including triple-H therapy. At admission, the neurologic and clinical conditions of patients were assessed using the Glasgow coma scale (GCS) scores and with the Hunt-Hess grading scale. The amount of SAH on CT image was evaluated using the Fisher grade. The clinical characteristics of the patients are summarized in
Table 1.
Application for angioplasty
When vasospasm was suspected, a brain CT or diffusion MRI scan was performed first to exclude other causes of clinical deficits, such as hydrocephalus, re-bleeding or cerebral infarction. Patients underwent angiography and IA nimodipine infusion if they showed at least one of the following conditions: 1) deterioration in the level of consciousness or clinical worsening based on GCS scores, 2) new motor weakness (arms or legs motor) or aphasia, 3) mean middle cerebral artery (MCA) flow velocity >150 cm/s with a Lindegaard ratio greater than 3, 4) a sudden rise of MCA velocity more than 50 cm/s/day. First IA nimodipine infusion was performed within 6 hours of symptomatic CV onset that was refractory to hemodynamic therapy in all cases.
Endovascular treatment of vasospasm
IA nimodipine infusions were performed by a neurosurgeon or neuroradiologist. The procedure was performed via a 5 F diagnostic catheter placed into the cervical segment on internal carotid artery. A dose of 5-15 mL nimodipine (1 mg/5 mL) was prepared after dilution with 15-45 mL physiologic saline. A slow, continuous infusion of the solution was achieved at a rate of 2 mL/min using an electric pump (nimodipine 0.1 mg/min). The dose of nimodipine infused intra-arterially was 1-3 mg per treated vessel. The total dose of nimodipine injected intra-arterially for a given patient was maintained within 5 mg and was determined by the severity of initial spasm, degree of vessel dilatation, and initial systemic blood pressure. To assess the angiographic effects of intra-arterially administered nimodipine, angiography was repeated 10 minutes after the end of the injection. Repetitive nimodipine infusion was performed if the patient had recurrent vasospasm based on the clinical symptoms, transcranial doppler (TCD) findings or angiography.
Clinical evaluation
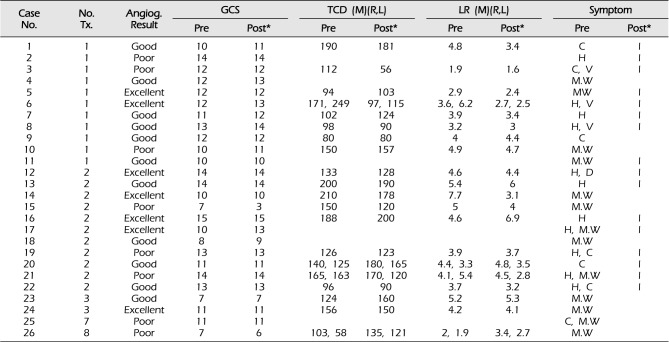
Based on the clinical charts, the GCS scores and clinical symptoms (including confusion, headache, vomiting, dizziness, and neurologic deficit) before and after each IA nimodipine infusion were assessed. Patient outcomes at 1 month and 3 months after angioplasty were assessed by the Glasgow outcome scale (GOS) score. Clinical outcomes of 26 patients treated with first IA nimodipine infusion are summarized in
Table 2.
TCD evaluation
Serial TCD studies of MCA blood flow velocity were performed daily from the day of admission. Mean MCA velocity (cm/sec) was recorded daily through a temporal window using a 2-MHz transducer (MultiDop P; DWL, Uberlingen, Germany).
Angiographic evaluation
The vessel diameters of the terminal(communicating) internal carotid artery (tICA), M1, M2, A1, and A2 portions were measured in millimeters on admission angiograms and on angiograms before and after IA nimodipine infusion. Vasospasm severity was determined based on the change in the vessel diameter between the initial and follow up angiography. Vasospasm grade was divided into grade one 0-25% (mild), grade two 26-50% (moderate), grade three 51-75% (severe), and grade four > 75% (critical). The initial admission arteriograms were used as a reference. The percentage of angiographic vasospasm was calculated by comparing vessel diameters in the initial angiogram with diameters of affected vessels measured prior to the IA nimodipine infusion. Percentage of angiographic improvement was calculated by comparing the diameter of affected and treated vessels before and after IA nimodipine infusion. An angiographic response was graded as poor if there was no improvement or the same vasospasm grade, good if a treated vessel segment improved by one angiographic grade (e.g., severe to moderate or moderate to mild), and excellent if vessels with marked spasm improved by two grades (eg, severe to mild). The number of affected vessel segments was determined, and vasospasm locations were classified as proximal (A1, M1, tICA), distal (A2, M2), and proximal and distal vasospasms.
Statistical methods
Statistical analysis was performed using IBM SPSS Statistics version 19.0 (SPSS Inc., Chicago, IL, USA). Continuous variables are expressed as means (standard deviation, SD). Independent sample T-test was used to compare with difference of continuous variables between single IA nimodipine infusion and multiple IA nimodipine infusion. For categorical variables, the chi-square test (Pearson chi square, Fisher's Exact test) was used. All tests were 2-tailed and p-values less than 0.05 were considered statistically significant.
RESULTS
Clinical characteristics
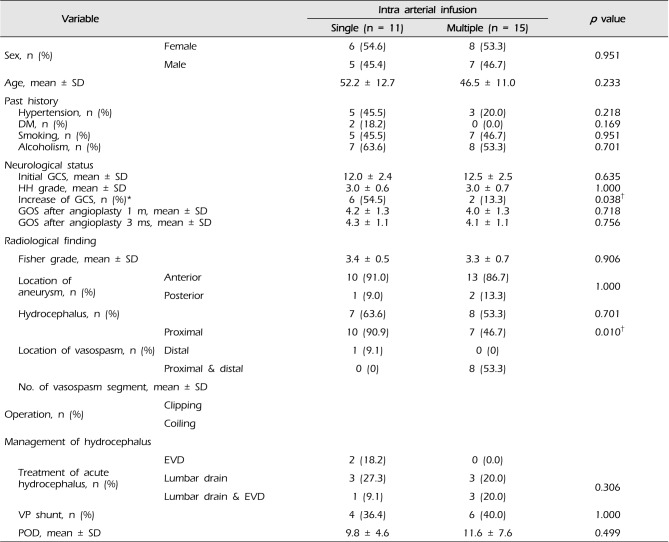
This study included 26 patients (12 men and 14 women) who underwent IA nimodipine infusions due to cerebral vasospasm with SAH after ruptured intracranial aneurysm. Eleven patients underwent a single IA nimodipine infusion (= single infusion) and 15 patients underwent more than two IA nimodipine infusions (= multiple infusion). The relationship between single or multiple infusion patients and clinical variable characteristics are shown in
Table 3. In 19 (73%) of 26 patients', aneurysms were treated by surgical clipping, whereas 7 patients (27%) underwent coil endovascular treatment. Patients experienced symptomatic CV from day 4 through to day 35 after SAH.
Improvement in clinical symptoms was observed after the IA nimodipine infusion in 8 of the 11 patients (72.7%) in single infusion patients. In multiple infusion patients, clinical improvement was observed after the first endovascular procedure in 8 patients (53.3%) (
Table 2). Six (54.5%) of the 11 patients had improved GCS scores during the 24 hours following vasospasm treatment with IA nimodipine infusion in single infusion patients, while 2 (13.3%) of the 15 patients showed GCS score improvement in multiple infusion patients. (
p = 0.038). The GOS scores of patients at 1 month and 3 months were not significantly different between single infusion patients and multiple infusion patients.
TCD findings
TCD recordings of MCA velocity data before and after angioplasty were available in 8 out of 11 patients in single infusion patients and 12 out of 15 patients in multiple infusion patients. In 6 of the total 26 patients, MCA velocity was not detected through the temporal windows using a 2-MHz transducer. Mean MCA velocities from 2 days pre-angioplasty to 3 days post-angioplasty are represented on time course curves (
Fig. 1). The mean MCA velocity before angioplasty gradually increased in both single and multiple infusion patients. On day one after the first angioplasty, the mean MCA velocity of single infusion patients decreased to 111.4 ± 38.5 cm/s, while the mean MCA velocity of multiple infusion patients slightly increased to 149.2 ± 32.1 cm/s. (
p = 0.018). For other days, no significant differences in the mean MCA velocity were detected between the single infusion patients and multiple infusion patients.
The mean MCA Lindegaard ratio values from day 2 pre-angioplasty to day 3 post-angioplasty are represented on time course curves (
Fig. 2). The graph configurations of single infusion patients and multiple infusion patients show similar patterns. The mean MCA Lindegaard ratio before angioplasty gradually increased and then began decreasing after the first angioplasty in both single and multiple infusion patients. There was only a statistical difference between single infusion patients and multiple infusion patients in the mean value of MCA Lindegaard ratio on the day after the first angioplasty (
p = 0.039). The mean MCA Lindegaard ratio on the first day after angioplasty was 3.1 ± 1.0 in single infusion patients and 4.3 ± 1.1 in multiple infusion patients. No significant difference between mean MCA Lindegaard ratios in single infusion patients and multiple infusion patients was detected on other days. In single infusion patients, three patients had decreased MCA velocity over 50 cm/s after the first angioplasty. However, no multiple infusion patients showed this finding (
p = 0.042).
Angiographic results
In single infusion patients, most CV occurred at the proximal vessels in 10 of 11 patients (90.9%), at the distal vessels in one patient (9.1%), and at both proximal and distal vessels in zero patients. In multiple infusion patients, the proximal and distal vessels were equally affected (at proximal vessels in 7 of 15 patients [46.7%], at distal vessels in zero patients, and at both proximal and distal vessels in 8 of 15 patients [53.3%]) (
p = 0.001). Of the 7 cases in which proximal vessels were affected in multiple infusion patients, CV occurred in bilateral cerebral hemispheres in five cases. There was only one case of bilateral proximal vessel involvement in single infusion patients (
p = 0.035). The average number of vasospastic vessel segments in single infusion patients (1.6 ± 0.5) was smaller than in multiple infusion patients (3.1 ± 1.0) (
p < 0.001) (
Table 3).
Before the first angioplasty, the average caliber of spastic vessels was 52.9% of the vessel caliber at admission in single infusion patients and 52.1% in multiple infusion patients. After the first angioplasty, spastic vessels dilated on average from 52.9 to 77.7% in single infusion patients and from 52.1 to 77.6% in multiple infusion patients. The immediate angiographic response was poor in 6 (33.3%) vessel segments, good in 10 (55.6%) vessel segments, and excellent in 2 (11.1%) of the 18 spastic vessel segments in single infusion patients. In multiple infusion patients, results were poor in 14 (30.4%) vessel segments, good in 22 (44.9%) vessel segments, and excellent in 10 (21.7%) of the 46 spastic vessel segments. There were no clinically evident complications or deaths related to the procedure.
DISCUSSION
Delayed ischemic neurological deficits caused by vasospasm may develop in 10 to 20% of patients.
1)5) Endovascular treatments with IA infusion of a vasodilator or mechanical angioplasty are employed as 'rescue' procedures for cerebral vasospasm following SAH that has not responded to maximal medical treatments. The goal of endovascular therapy is to improve cerebral blood flow, leading to reduced risk of infarction.
Papaverine, verapamil, nimodipine, and nicardipine have been used as IA vasodilators to treat patients with symptomatic vasospasm refractory to medical therapy.
1) Papaverine, verapamil, nimodipine, and nicardipine have half-lives of 2, 7, 9, and 16 hours, respectively.
14) Their duration of action on spastic vessels varies, but the exact durability of these substances is unknown. No other calcium channel antagonists are superior in terms of safety or efficacy.
IA infusion of nimodipine has been proven as an effective agent on clinical outcome after SAH in several clinical trials.
2)3)4)6)7)8) IA nimodipine infusion has been used as a routine endovascular procedure for medically refractory CV for many years. Although nimodipine is a potent drug for vessel relaxation, the side effect of systemic hypotension precluded sufficient doses. For this reason, incomplete treatment or recurrence frequently occurred after single IA nimodipine infusions. IA nimodipine infusions then had to be repeated until there was no further evidence of vasospasm in the clinical symptoms, TCD findings, or angiography.
TCD findings
There are many published studies about using TCD for CV diagnosis after SAH. However, data is lacking on TCD findings before and after endovascular treatment for medically refractory CV, especially in terms of serial follow-up. Kim et al. observed decreased velocities in 29 of 33 available TCD data after IA nimodipine infusion.
7) There was no increase in velocity after IA nimodipine infusion in their cases. Musahl et al. observed a steady decrease in blood flow velocity on TCD in all patients after onset of IA infusion.
10) Biondi et al. observed decreased MCA velocities by at least 20 cm/s in seven cases, slightly changed velocities in 20 patients, and increased velocities by at least 20 cm/s in 3 of 30 procedures after IA nimodipine infusion.
2) Ott et al. reported no reliable changes in flow velocities.
13)
TCD has a high positive predictive value and low negative predictive value regarding MCA spasms.
9) In our study, TCD also showed a low negative predictive value. MCA velocity did not show evidence of CV in 6 of 26 (23.0%) of the total patients even though their angiography showed definite CV. However, different TCD patterns were identified for single infusion patients and multiple infusion patients using the available serial follow-up TCD measurements. Significant differences in mean MCA velocity and mean MCA Lindegaard ratio the first day, after the first angioplasty were detected between the single infusion patients and multiple infusion patients. In multiple infusion patients, no patients showed decreased MCA velocity over 50 cm/s on the day after angioplasty while 3 of the single infusion patients showed decreased MCA velocity over 50 cm/s on day one. Based on our study, repeated IA nimodipine may be continued when mean MCA velocity does not show a decreasing trend (over 50 cm/s) and the Lindegaard ratio is over 4.3 after the IA nimodipine infusion on day one.
Single IA nimodipine infusion patients
Kim et al. reported a significant positive correlation between the degree of blood vessel expansion and the improvement in clinical symptoms.
8) However, Biondi et al. noted clinical improvement after IA nimodipine infusions without notable vascular dilatation in 7 (37%) of 19 patients.
2) Though the effect of IA nimodipine appears to be more enduring than that of IA papaverine, the efficacy of IA nimodipine is known to be temporary. Hänggi et al. investigated the effect and duration of action of IA nimodipine using perfusion CT.
4) They demonstrated that the effect of IA nimodipine lasts for, at most, 24 hours by revealing that the reduced time to peak and mean transit times occurred 1 day after intervention. Therefore, a single IA nimodipine infusion can save only patients at the peak of vasospasm because these patients tend to improve during the following days.
4)
In the present study, single infusion patients had less severe CV. Vasospasm occurred focally; mostly in the proximal vessel of unilateral cerebral hemispheres. Improvement in GCS scores (54.5%) was significantly better in single infusion patients than multiple infusion patients (13.3%). In single infusion patients, three patients showed clinical or GCS score improvement although their immediate angiographic responses were poor. One possible explanation is that because IA nimodipine acts on the more distal parts of cerebral vasculature, cerebral circulation time might have improved without notable angiographic changes.
12)
Multiple IA nimodipine infusion patients
In multiple infusion patients, vasospasm occurred more diffusely, bilaterally, and distally. The number of spastic vessel segments was associated with the necessity for multiple infusions. Improvement in GCS score after the first angioplasty was poorer in multiple infusion patients than single infusion patients. Only 2 of 15 patients (13.3%) showed GCS score improvement after first angioplasty.
After the first angioplasty, spastic vessels dilated from 52.1 to 77.6% on average. However, treated vessels narrowed from 77.6 to 56.0% on the following day. Spastic vessels dilated again from 56.0 to 77.3% after the second angioplasty. Immediate angiographic response to first and second infusions did not differ. It was difficult to discern if the results were purely due to incomplete treatment or vasospasm recurrence because patients had multiple vasospastic vessel segments and each vessel segment responded differently to IA nimodipine infusion. Some vessel segments showed vasospasm recurrence while other segments showed incomplete dilatation in the same patient.
Multiple infusion patients are likely in more critical situations than single infusion patients. Multiple infusion patients required more careful monitoring and successive IA nimodipine infusions to recover from critical CV. After follow-up of 1-3 months, there was no significant difference in GOS between the single infusion patients and multiple infusion patients.
If the demographic and clinical factors related to multiple IA infusion are known, the need for repetitive endovascular treatment can be predicted. Potential candidates for multiple infusions can be intensively monitored and consecutive treatment can be efficiently and systematically performed. This study may be helpful in decision-making regarding repetition of IA nimodipine infusion.
This study has some limitations. Data collection was retrospective. The number of patients was relatively small, which may lead to statistical misinterpretation. The primary goal of endovascular treatment for symptomatic vasospasm is to increase cerebral blood flow to prevent infarction, yet infarction rate due to vasospasm was not included.
CONCLUSION
Based on our study, following factors were related with multiple IA nimodipine infusions: 1) no improvement in GCS after the IA nimodipine infusion; 2) no decrease of MCA velocity on TCD over 50 cm/s or Lindegaard ratio over 4.3 after the IA nimodipine infusion; 3) distal, bilateral, or diffuse involvement of CV.
Fig. 1
Change of mean MCA velocity in single infusion patients (solid line) and multiple infusion patients (dotted line). MCA = middle cerebral artery. *Solid line = 111.4 ± 38.5 cm/s, dotted line = 149.2±32.1 cm/s. (p = 0.018). Statistically significant difference (p < 0.05).
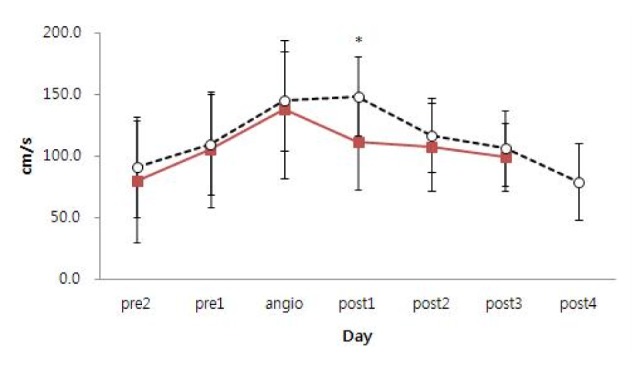
Fig. 2
Change of mean MCA Lindegaard ratio (LR) in single infusion patients (solid line) and multiple infusion patients (dotted line). MCA = middle cerebral artery; LR = Lindegaard ratio. *Solid line = 3.1 ± 1.0, dotted line = 4.3 ± 1.1 (p = 0.039). Statistically significant difference (p < 0.05).
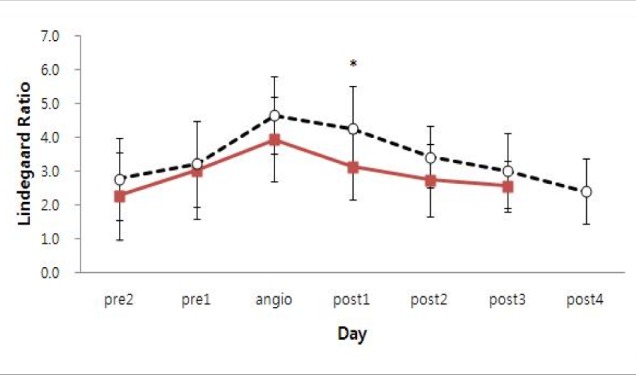
Table 1
Baseline characteristics of the patients enrolled in this study (n = 26)
|
Case No. |
Sex |
Age |
HH Gr |
Fish Gr |
Loc. Of An. |
An. Treat. |
After SAH |
Vasospasm Location |
1 m. G |
3 m. G |
|
1 |
F |
44 |
4 |
4 |
Acom |
Clipping |
12 |
L M1, A1 |
5 |
5 |
|
2 |
F |
36 |
2 |
3 |
L AnCh |
Coiling |
11 |
L M1, A1 |
5 |
5 |
|
3 |
F |
49 |
3 |
4 |
L ICAB |
Coiling |
9 |
L M1, A1 |
5 |
5 |
|
4 |
F |
63 |
3 |
4 |
Acom |
Clipping |
5 |
R A1 |
2 |
3 |
|
5 |
F |
74 |
3 |
3 |
L MCA |
Clipping |
7 |
L M1 |
2 |
3 |
|
6 |
M |
35 |
3 |
3 |
R VA |
Coiling |
22 |
B M1 |
5 |
5 |
|
7 |
M |
42 |
3 |
3 |
L Pcom |
Clipping |
5 |
L M1 |
5 |
5 |
|
8 |
M |
51 |
3 |
3 |
Acom |
Clipping |
8 |
L M1, A1 |
5 |
5 |
|
9 |
M |
52 |
2 |
3 |
Acom |
Clipping |
11 |
R M1, A1 |
5 |
5 |
|
10 |
M |
66 |
4 |
4 |
R MCA |
Clipping |
9 |
R M1, A1 |
3 |
4 |
|
11 |
F |
62 |
3 |
3 |
Acom |
Clipping |
9 |
L M2 |
2 |
2 |
|
12 |
F |
30 |
3 |
3 |
R DorW |
Coiling |
10 |
R M1, M2, A1 |
5 |
5 |
|
13 |
F |
47 |
2 |
3 |
R MCA |
Clipping |
6 |
R M1, M2, A1 |
5 |
5 |
|
14 |
F |
50 |
3 |
4 |
BT |
Coiling |
9 |
L M1, M2, A1 |
4 |
4 |
|
15 |
F |
52 |
4 |
4 |
Acom |
Clipping |
6 |
R M1, A1 |
1 |
1 |
|
16 |
F |
55 |
3 |
3 |
Acom |
Clipping |
10 |
L M1, M2, A1 |
5 |
5 |
|
17 |
F |
58 |
3 |
2 |
L MCA |
Clipping |
16 |
R M1, M2 |
3 |
3 |
|
18 |
F |
68 |
4 |
4 |
Acom |
Clipping |
8 |
B M1, L A1 |
4 |
4 |
|
19 |
M |
34 |
3 |
4 |
Acom |
Clipping |
8 |
L M1, A1 |
4 |
5 |
|
20 |
M |
37 |
3 |
3 |
Acom |
Clipping |
9 |
B M1, A1, tICA |
5 |
5 |
|
21 |
M |
44 |
3 |
4 |
R pcom |
Clipping |
12 |
B M1, A1 |
5 |
5 |
|
22 |
M |
54 |
3 |
4 |
R MCA |
Clipping |
12 |
L M1, M2, A1 |
5 |
5 |
|
23 |
F |
55 |
2 |
3 |
R DorW |
Coiling |
35 |
R M2, A1 |
2 |
3 |
|
24 |
M |
44 |
2 |
3 |
Acom |
Clipping |
4 |
L M1, M2, A1 |
4 |
4 |
|
25 |
M |
39 |
4 |
4 |
BT |
Coiling |
9 |
B M1, A1 |
4 |
4 |
|
26 |
M |
30 |
3 |
2 |
R DorW |
Clipping |
20 |
B M1, R A1 |
2 |
3 |
Table 2
Clinical outcomes of 26 patients treated with intra arterial nimodipine infusion
|
Case No. |
No. Tx. |
Angiog. Result |
GCS |
TCD (M)(R,L) |
LR (M)(R,L) |
Symptom |
|
Pre |
Post*
|
Pre |
Post*
|
Pre |
Post*
|
Pre |
Post*
|
|
1 |
1 |
Good |
10 |
11 |
190 |
181 |
4.8 |
3.4 |
C |
I |
|
2 |
1 |
Poor |
14 |
14 |
|
|
|
|
H |
I |
|
3 |
1 |
Poor |
12 |
12 |
112 |
56 |
1.9 |
1.6 |
C, V |
I |
|
4 |
1 |
Good |
12 |
13 |
|
|
|
|
M.W |
|
|
5 |
1 |
Excellent |
12 |
12 |
94 |
103 |
2.9 |
2.4 |
MW |
I |
|
6 |
1 |
Excellent |
12 |
13 |
171, 249 |
97, 115 |
3.6, 6.2 |
2.7, 2.5 |
H, V |
I |
|
7 |
1 |
Good |
11 |
12 |
102 |
124 |
3.9 |
3.4 |
H |
I |
|
8 |
1 |
Good |
13 |
14 |
98 |
90 |
3.2 |
3 |
H, V |
I |
|
9 |
1 |
Good |
12 |
12 |
80 |
80 |
4 |
4.4 |
C |
|
|
10 |
1 |
Poor |
10 |
11 |
150 |
157 |
4.9 |
4.7 |
M.W |
|
|
11 |
1 |
Good |
10 |
10 |
|
|
|
|
M.W |
I |
|
12 |
2 |
Excellent |
14 |
14 |
133 |
128 |
4.6 |
4.4 |
H, D |
I |
|
13 |
2 |
Good |
14 |
14 |
200 |
190 |
5.4 |
6 |
H |
I |
|
14 |
2 |
Excellent |
10 |
10 |
210 |
178 |
7.7 |
3.1 |
M.W |
|
|
15 |
2 |
Poor |
7 |
3 |
150 |
120 |
5 |
4 |
M.W |
|
|
16 |
2 |
Excellent |
15 |
15 |
188 |
200 |
4.6 |
6.9 |
H |
I |
|
17 |
2 |
Excellent |
10 |
13 |
|
|
|
|
H, M.W |
I |
|
18 |
2 |
Good |
8 |
9 |
|
|
|
|
M.W |
|
|
19 |
2 |
Poor |
13 |
13 |
126 |
123 |
3.9 |
3.7 |
H, C |
I |
|
20 |
2 |
Good |
11 |
11 |
140, 125 |
180, 165 |
4.4, 3.3 |
4.8, 3.5 |
C |
I |
|
21 |
2 |
Poor |
14 |
14 |
165, 163 |
170, 120 |
4.1, 5.4 |
4.5, 2.8 |
H, M.W |
I |
|
22 |
2 |
Good |
13 |
13 |
96 |
90 |
3.7 |
3.2 |
H, C |
I |
|
23 |
3 |
Good |
7 |
7 |
124 |
160 |
5.2 |
5.3 |
M.W |
|
|
24 |
3 |
Excellent |
11 |
11 |
156 |
150 |
4.2 |
4.1 |
M.W |
|
|
25 |
7 |
Poor |
11 |
11 |
|
|
|
|
C, M.W |
|
|
26 |
8 |
Poor |
7 |
6 |
103, 58 |
135, 121 |
2, 1.9 |
3.4, 2.7 |
M.W |
|
Table 3
The relationship between single or multiple infusion patients and clinically various characteristics
|
Variable |
|
Intra arterial infusion |
p value |
|
Single (n = 11) |
Multiple (n = 15) |
|
Sex, n (%) |
Female |
6 (54.6) |
8 (53.3) |
0.951 |
|
Male |
5 (45.4) |
7 (46.7) |
|
Age, mean ± SD |
|
52.2 ± 12.7 |
46.5 ± 11.0 |
0.233 |
|
Past history |
|
|
|
|
|
Hypertension, n (%) |
|
5 (45.5) |
3 (20.0) |
0.218 |
|
DM, n (%) |
|
2 (18.2) |
0 (0.0) |
0.169 |
|
Smoking, n (%) |
|
5 (45.5) |
7 (46.7) |
0.951 |
|
Alcoholism, n (%) |
|
7 (63.6) |
8 (53.3) |
0.701 |
|
Neurological status |
|
|
|
|
|
Initial GCS, mean ± SD |
|
12.0 ± 2.4 |
12.5 ± 2.5 |
0.635 |
|
HH grade, mean ± SD |
|
3.0 ± 0.6 |
3.0 ± 0.7 |
1.000 |
|
Increase of GCS, n (%)*
|
|
6 (54.5) |
2 (13.3) |
0.038†
|
|
GOS after angioplasty 1 m, mean ± SD |
4.2 ± 1.3 |
4.0 ± 1.3 |
0.718 |
|
GOS after angioplasty 3 ms, mean ± SD |
4.3 ± 1.1 |
4.1 ± 1.1 |
0.756 |
|
Radiological finding |
|
|
|
|
|
Fisher grade, mean ± SD |
|
3.4 ± 0.5 |
3.3 ± 0.7 |
0.906 |
|
Location of aneurysm, n (%) |
Anterior |
10 (91.0) |
13 (86.7) |
1.000 |
|
Posterior |
1 (9.0) |
2 (13.3) |
|
Hydrocephalus, n (%) |
|
7 (63.6) |
8 (53.3) |
0.701 |
|
Location of vasospasm, n (%) |
Proximal |
10 (90.9) |
7 (46.7) |
0.010†
|
|
Distal |
1 (9.1) |
0 (0) |
|
|
Proximal & distal |
0 (0) |
8 (53.3) |
|
|
No. of vasospasm segment, mean ± SD |
|
|
|
|
Operation, n (%) |
Clipping |
|
|
|
|
Coiling |
|
|
|
|
Management of hydrocephalus |
|
|
|
|
|
Treatment of acute hydrocephalus, n (%) |
EVD |
2 (18.2) |
0 (0.0) |
0.306 |
|
Lumbar drain |
3 (27.3) |
3 (20.0) |
|
Lumbar drain & EVD |
1 (9.1) |
3 (20.0) |
|
VP shunt, n (%) |
|
4 (36.4) |
6 (40.0) |
1.000 |
|
POD, mean ± SD |
|
9.8 ± 4.6 |
11.6 ± 7.6 |
0.499 |









 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download