Abstract
Rupture of spontaneous dissecting aneurysms of the middle cerebral artery (MCA) is rare and its etiology remains obscure, although the risk of rebleeding is greater than with saccular aneurysms. Most reports concerning the treatment of a ruptured dissecting aneurysm of the anterior circulation involve surgical trapping or wrapping. Here, we report on a case of an MCA dissecting rupture treated with endovascular procedures. A 22-year-old female presented with sudden stuporous mental change following severe headache and left side hemiparesis. A computed tomography scan showed a diffuse subarachnoid hemorrhage and diffusion MR showed diffusion restriction at the right putamen and internal capsule. A 3-hour follow-up digital subtraction angiography (DSA) showed a dissecting aneurysm, which was not seen on an initial DSA. A stent assisted coil embolization was performed and double stents were applied to achieve flow diversion effects. A small remnant area of the dissecting aneurysm had disappeared at 60-day and was not observed on 12-month follow-up DSA.
Since they were first reported by Kunze and Schiefer in 1971, spontaneous dissecting aneurysms localized in the anterior circulation appear to be rare.10) The etiology and prognosis of dissecting aneurysms remain obscure. The natural history of ruptured dissecting aneurysms is ill-defined but the risk of early rebleeding is significant compared to that of the ruptured saccular aneurysms.2)4)6)9) In addition, ruptured intracranial dissecting aneurysms in nonvertebral locations are rare and surgical options are limited with poor prognosis.12) Diagnosis of a vertebral artery dissecting aneurysm is classically represented as an angiographic pearl and string sign, the dilatation of the lumen adjacent to a stenotic segment.17) However, the Pearl-and-string sign is attenuated in other locations and may not be recognizable.6)9) In addition, trapping without bypass may not be considered as a first line operation for a nonvertebral location.2)12)
Most intracranial dissecting aneurysms of the vertebrobasilar system are associated with subarachnoid hemorrhage (SAH), whereas those of the carotid system typically present with cerebral infarction caused by arterial stenosis and occlusion. Only 14% of intracranial dissecting aneurysms causing hemorrhage occur in the anterior circulation.4)17) Here we report on a case of a spontaneous SAH resulting from the rupture of a dissection in the middle cerebral artery (MCA), which was treated with a stent and coil in an attempt to preserve arterial continuity.
A 22-year-old female suddenly developed stuporous mental change following a severe headache and left side hemiparesis (motor grade 3). The patient did not have clear history of trauma and there was no external wound. She had no other previous medical history such as hypertension, infection sign and initial blood pressure was 94/63. Computed tomography (CT) performed by a local physician showed SAH and the Fisher grade was assessed as 3 (Fig. 1A), although no definite saccular aneurysm was observed on CT angiography (Fig. 1B). Digital subtraction angiography (DSA) performed 5 hours after initial symptoms by a local physician showed slight irregular dilatation (but not definite aneurysmal dilatation) in the right middle cerebral artery (MCA) around the anterior temporal artery (Fig. 1C). A local physician was unsure of the cause of the SAH and therefore referred the patient to us after DSA (5 hours from symptom onset). On admission to our hospital, her Glasgow Coma Scale score was 9 and Hunt Hess grade was 4. Diffusion-weighted magnetic resonance imaging (DW-MRI) showed diffusion restriction at the right putamen and internal capsule (Fig. 1D).
DSA was performed again at our hospital, 3 hours after a previous DSA (8 hours after symptom onset). The new DSA showed definite aneurysmal dilatation on the postero-inferior aspect of the right MCA (Fig. 2A, B). The diagnosis was a ruptured right MCA dissecting aneurysm associated with a cerebral infarction, with unknown etiology. The patient underwent endovascular treatment on the day of admission to prevent repeated hemorrhage. Stent-assisted coil embolization was planned for achievement of flow diversion effects. A first stent was deployed at M1 (Enterprise 4.5 × 22 mm, Codman, Raynham, MA, USA) after selecting the aneurysm with another microcatheter. Detachable coils were inserted into the aneurysm (Deltaplush, 2 × 3 mm, Codman, Raynham, MA, USA and Target 360 Ultrasoft, 1.5 × 2 mm and 1 × 2 mm, Stryker, Natick, MA, USA). An additional intracranial stent was overlapped at M1 (Enterprise, 4.5 × 28 mm, Codman, Raynham, MA, USA) and a coil was finally inserted (Target Helical Ultrasoft, 1 × 2 mm, Stryker, Natick, MA, USA) (Fig. 2C, D).
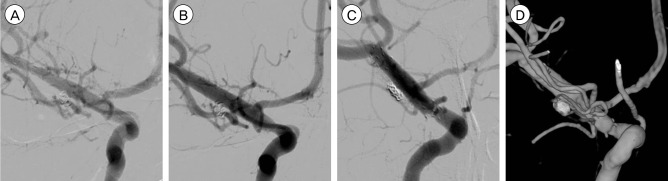
The postoperative course was benign and mental status was recovered. Left hemiparesis was also recovered and returned to normal within a week. DSA was performed 4 times after endovascular therapy within four weeks. Fortunately, the fourth follow up DSA at 30 days showed no further enlargement of the aneurysm or dissection (Fig. 3B), although a short-term (after 7 days) follow-up DSA showed slight enlargement of the aneurysmal neck (Fig. 3A). On long-term follow-up angiography performed at 6 and 12 months after therapy the remnant aneurysm had disappeared (Fig. 3C, D). There were no irregularities on the vessel wall and endothelialization of the dissecting area was considered successful.
Dissecting intracranial aneurysms as the source of subarachnoid hemorrhage are relatively rare and treatment options are limited. The etiology and natural history are still not known. Our review of the literature found 25 cases of MCA dissecting aneurysms after 1990.2) According to the literature, MCA dissecting aneurysms occurred in young patients in contrast to aneurysms of the vertebrobasilar circulation. In this review all patients were treated surgically by wrapping, clipping, and excision, with bypasses in 19 cases, whereas 6 patients were treated medically. The mortality rate of patients who received conservative treatment was 50% (3/6).
The appropriate management of patients with dissecting aneurysms remains controversial and the indications for surgical treatment of MCA dissecting aneurysms are not well-defined. However, there is a consensus that surgical treatment, such as wrapping or trapping with arterial reconstruction, may be appropriate in the presence of SAH, since subsequent bleeding often leads to poor outcomes. Ohkuma described a series of dissecting aneurysms in the intracranial carotid circulation with SAH.15) Rebleeding of aneurysms occurred in half of the patients within 14 days, suggesting that early surgery is critical for preventing rebleeding in cases of dissecting aneurysms of the MCA.
In vertebral dissecting rupture, complete occlusion of a dissected segment by trapping or coil embolization is the first choice when contralateral VA flow exists.7)9)11)13)14) However, reports on use of endovascular treatment of MCA dissecting aneurysms are limited. Proximal occlusion or trapping without an efficient bypass pose a significant risk of MCA territory infarct. Severe hemiparesis or hemiplegia by lenticulostriate artery infarction is inevitable when M1 is occluded by trapping or coil embolization, although there is successful bypass to the cortical MCA. Preventing rupture and stabilizing the dissected wall with preservation of MCA flow, while allowing successful healing by endothelialization, seems to be the optimal therapy for ruptured dissecting MCA aneurysm. Recently, flow-diverting stents, such as the Pipeline, have been reported as a satisfactory endovascular option in treatment of giant or dissecting aneurysms to preserve original arterial flow.1)3)5)16) However, there are still some concerns regarding cost-effectiveness, fractures, increases in intra-aneurysmal pressure due to check-valve effects, or perforator ischemia, especially in the circumstance of numerous small perforators, as found with the lenticulostriate artery.
To the best of our knowledge, this is the first report of endovascular therapy alone in treatment of a ruptured MCA dissecting aneurysm. Stent deployment and coil embolization is not a concept of compact coil packing of dissected aneurysm but a flow diversion with stents and decreased blood flow into the dissecting rupture site. The rupture site is very fragile and excessive coil packing should be avoided due to risk of spreading on the dissected wall. It seems to be suitable that coils 'flow in' the aneurysm. Stent deployment in the dissection area is for flow diversion effects by stent struts and closed cell type stents are more efficient than the open cell type. Double or triple stent deployment should have more prominent flow diversion effects than single stent. Recently, double or triple stents without coiling were utilized in some dissecting rupture cases. However, stents with coiling were more effective in flow diversion than stents alone. When stents are deployed, it seems that the space should be prepared for M1 trapping and EC-IC bypass, as it is desirable not to pass the distal stent tip through the MCA bifurcation, if possible.
In patients with SAH and cerebral infarction without apparent causative complications, the presence of a dissecting aneurysm should be considered even in the absence of apparent abnormalities on the initial angiogram.8) In cases of a suspected dissecting aneurysm, diagnostic examinations and serial angiographic studies should be performed immediately due to the potential for occurrence of dynamic changes over a short period of time.8) The patient in our case also had angiographic progression of a dissecting aneurysm within only three hours. Even after successful endovascular therapy, close observation and short-term serial angiography follow-up are essential due to the probability of progression of dissection, which is often. Although it is the most accurate diagnostic tool available, conventional angiography is invasive and frequently difficult to perform. In practical terms, simple skull X-rays to observe changes in the coil mesh configuration can serve as good accessorial tools for patient follow-up.
We report on endovascular coil embolization with stents in ruptured MCA dissection, which provides a good method of flow diversion, while preserving MCA flow and M1 perforators. However, surgeons always prepare additional surgical or endovascular therapy when dissection is aggravated. Close observation with frequent image follow-up is essential in ruptured dissection, even after successful treatment.
References
1. Chan RS, Mak CH, Wong AK, Chan KY, Leung KM. Use of the pipeline embolization device to treat recently ruptured dissecting cerebral aneurysms. Interv Neuroradiol. 2014; Jul-Aug. 20(4):436–441. PMID: 25207906.

2. Chuang MJ, Lu CH, Cheng MH. Management of middle cerebral artery dissecting aneurysm. Asian J Surg. 2012; 1. 35(1):42–48. PMID: 22726563.

3. Fischer S, Perez MA, Kurre W, Albes G, Bazner H, Henkes H. Pipeline embolization device for the treatment of intra- and extracranial fusiform and dissecting aneurysms: initial experience and long-term follow-up. Neurosurgery. 2014; 10. 75(4):364–374. discussion 374PMID: 24871140.
4. Friedman AH, Drake CG. Subarachnoid hemorrhage from intracranial dissecting aneurysm. J Neurosurg. 1984; 2. 60(2):325–334. PMID: 6693960.

5. Gong D, Yan B, Dowling R, Mitchell P. Successful treatment of growing basilar artery dissecting aneurysm by pipeline flow diversion embolization device. J Stroke Cerebrovasc Dis. 2014; 7. 23(6):1713–1716. PMID: 24389375.

6. Hosoda K, Fujita S, Kawaguchi T, Shose Y, Yonezawa K, Shirakuni T, et al. Spontaneous dissecting aneurysms of the basilar artery presenting with a subarachnoid hemorrhage. Report of two cases. J Neurosurg. 1991; 10. 75(4):628–633. PMID: 1885981.
7. Kawaguchi S, Sakaki T, Tsunoda S, Morimoto T, Hoshida T, Kawai S, et al. Management of dissecting aneurysms of the posterior circulation. Acta Neurochir (Wien). 994; 3. 131(1-2):26–31. PMID: 7709782.

8. Kim YW, Yoo SH, Kim SR, Kim SD, Park IS, Baik MW. Dissecting aneurysm at the A1 segment of the anterior cerebral artery manifesting as subarachnoid hemorrhage: two case reports. Korean J Cerebrovasc Surg. 2005; 12. 7(4):324–328.
9. Kitanaka C, Sasaki T, Eguchi T, Teraoka A, Nakane M, Hoya K. Intracranial vertebral artery dissections: clinical, radiological features, and surgical considerations. Neurosurgery. 1994; 4. 34(4):620–626. discussion 266-7PMID: 8008158.
10. Kunze S, Schiefer W. Angiographic demonstration of a dissecting aneurysm of the middle cerebral artery. Neuroradiology. 1971; 9. 2(4):201–206. PMID: 5164128.

11. Kurata A, Ohmomo T, Miyasaka Y, Fujii K, Kan S, Kitahara T. Coil embolization for the treatment of ruptured dissecting vertebral aneurysms. AJNR Am J Neuroradiol. 2001; 1. 22(1):11–18. PMID: 11158881.
12. Kurino M, Yoshioka S, Ushio Y. Spontaneous dissecting aneurysms of anterior and middle cerebral artery associated with brain infarction: a case report and review of the literature. Surg Neurol. 2002; 6. 57(6):428–436. discussion 436-8PMID: 12176212.
13. Manabe H, Hatayama T, Hasegawa S, Islam SM, Suzuki S. Coil embolisation for ruptured vertebral artery dissection distal to the origin of the posterior inferior cerebellar artery. Neuroradiology. 2000; 5. 42(5):384–387. PMID: 10872163.

14. Manabe H, Ohkuma H, Fujita S, Suzuki S. Coil embolization of ruptured vertebral dissection in acute stage with interlocking detachable coils. Surg Neurol. 1997; 5. 47(5):476–480. PMID: 9131033.

15. Ohkuma H, Suzuki S, Shimamura N, Nakano T. Dissecting aneurysms of the middle cerebral artery: neuroradiological and clinical features. Neuroradiology. 2003; 3. 45(3):143–148. PMID: 12684715.

16. Prasad V, Gandhi D, Jindal G. Pipeline endovascular reconstruction of traumatic dissecting aneurysms of the intracranial internal carotid artery. J Neurointerv Surg. 2014; 12. 6(10):e48. PMID: 24353328.

17. Yamaura A, Watanabe Y, Saeki N. Dissecting aneurysms of the intracranial vertebral artery. J Neurosurg. 1990; 2. 72(2):183–188. PMID: 2404089.

Fig. 1
Initial radiologic images. (A) Plain computed tomography (CT) scan shows diffuse subarachnoid hemorrhage. (B) CT angiogram shows no definite saccular aneurysm. (C) First digital subtraction angiogram (DSA) shows no definite saccular aneurysm except for a slight irregular dilatation of the posterior aspect of the right proximal M1. (D) Diffusion-wighted MR image shows diffusion restriction in the right putamen and posterior part of the internal capsule.
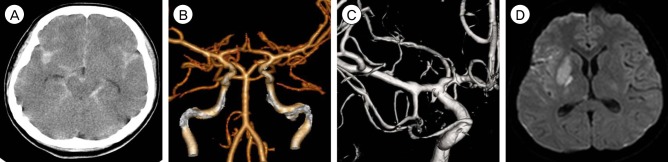
Fig. 2
(A, B) Follow-up digital subtraction angiogram (DSA) (3 hours after the previous DSA) shows saccular aneurysmal dilatation at the postero-inferior aspect of the right middle cerebral artery (M1), which is not seen on the previous angiogram and is observed at the same location of slight irregular dilatation of M1 on the previous angiogram. The aneurysm was measured as 3.0 × 2.3 mm in size with a neck size of 2.7 mm. (C, D) Coil embolization and double stent deployment were performed.
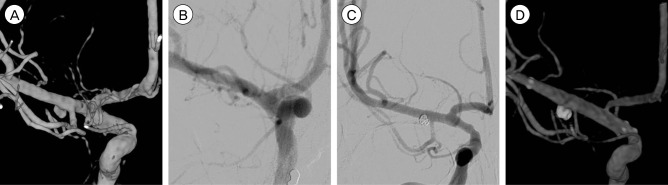
Fig. 3
Follow-up digital subtraction angiogram (DSA) after stent-assisted coil embolization. (A) The first follow-up angiogram performed 7 days after intervention shows no definite interval change. (B) The fourth follow-up angiogram, 30 days after intervention, shows the disappearance of the previous dilatation of the aneurysmal neck. Angiograms (C and D) one year after intervention, shows no further aneurysmal dilatation around the coiled site. The middle cerebral artery flow is completely separated from the coils and stents on angiography, as a result of successful healing and endothelialization.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download