Abstract
Pial arteriovenous fistulas (pAVF) are rare vascular lesions consisting of one or more arterial connections to a single venous channel without any intervening nidus of vessels or capillaries.
Case 1: A 65-year-old woman with a complaint of headache and left hand paresthesia was referred to us. Magnetic resonance imaging showed a large saccular lesion with signal void in the posterior part of the right sylvian fissure and catheter angiography showed a giant venous aneurysm fed by one branch of the middle cerebral artery (MCA) and draining into the vein of Trolard.
Case 2: A 12-year-old boy was transferred to our hospital with a history of sudden loss of consciousness and hemiplegia. Brain computed tomography revealed a massive hemorrhagic mass in the right hemisphere and cerebral angiography showed a pAVF with a large aneurysmal varix, which was fed by multiple branches of the right MCA and draining into the superior sagittal sinus.
Both patients underwent craniotomy and after ligation of vascular connections, aneurysmal varices were removed completely. Surgical resection can be a safe method for treatment of pAVFs, particularly in those with large varices.
Intracranial pial arteriovenous fistulas (pAVF) are rare vascular lesions accounting for only 1.6% of all vascular malformations of the brain.9)28) These lesions have a single or multiple arterial connections to a single venous channel without any intervening nidus of vessel or capillaries. pAVF differs from dural AVF in that the arterial supply is derived from pial or cortical arterial vessels and the location is not within the dural leaflets.11) Natural course of pAVFs is not favorable and most require an intervention.18) Due to their rarity, there is no consensus regarding an optimal method of treatment. Most authors have reported simple disconnection of an arteriovenous shunt by either microsurgery or endovascular embolization without surgical resection, however, in large ones, pressure effect cannot be eliminated by use of these methods. In this report, we describe two cases of pAVF associated with giant varices that underwent surgical resection.
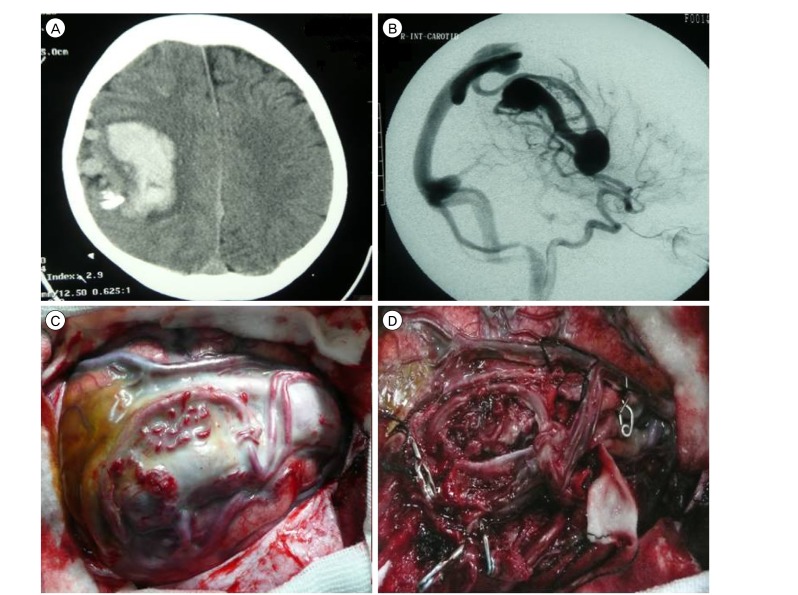
A 65-year-old woman with a complaint of headache and paresthesia in the left upper extremity was transferred to our hospital. The onset of symptoms dated back to one month ago. She had a history of hypertension and diabetes mellitus but had no history of head injury. Neurological examination showed normal motor function of extremities. Brain computed tomography (CT) and magnetic resonance imaging (MRI) showed a large saccular lesion in the posterior part of the right sylvian fissure with signal void and small calcifications in its wall (Fig. 1A and 1B). CT angiography and catheter angiography showed a giant venous aneurysm fed by one branch of the middle cerebral artery (MCA) and draining into the vein of Trolard, which was consistent with single-channel pAVF (Fig. 1C).
We attributed the patient's symptoms mainly to the mass effect of the aneurysm and decided to perform resection of the lesion. After right frontotemporoparietal craniotomy, the sylvian fissure was opened widely. Venous aneurysm and its feeding artery and draining vein were detected; one clip was placed on a feeding artery and another on the draining vein just close to the aneurysm. Blood in the lesion was aspirated and the aneurysm collapsed. It was dissected from surrounding tissues through the arachnoidal plane and en bloc removal was finally achieved (Fig. 1D). Microscopic examination of the resected specimen revealed fragments of hyalinized and calcified vessles with myxoid changes (Fig. 1E).
Her postoperative course was uncomplicated. At a follow-up visit three months after surgery, she had no headache and neurological examination was normal.
A 12-year-old right-handed boy was transferred to our hospital after two weeks of admission in a local hospital due to sudden loss of consciousness and hemiplegia. On arrival, the patient was drowsy with left-sided severe hemiparesis (motor power = 2/5). Brain CT scan showed a huge heterogeneous hemorrhagic mass in the right hemisphere as well as peripheral edema (Fig. 2A). Cerebral digital subtraction angiography (DSA, Fig. 2B) showed a pAVF with a large aneurysmal varix, which was fed by multiple branches of the right MCA and draining to the superior sagittal sinus. Twenty eight days after the onset of symptoms and with remarkable recovery in neurologic status (motor power = 4/5), he underwent right temporoparietal craniotomy. After dural opening and obtaining proximal control, the varix was carefully dissected. We detected three large and four small arteries entering the lesion. Arterial feeders were obliterated, just proximal to the varix and the solitary draining vein was tied and cut. The varix was finally removed in one piece (Fig 2C and 2D). During the 48 hour post operative period, he was under mild sedation and hypotension was induced with intravenous infusion of midazolam and labetalol. Postoperative course was uneventful, and brain CT scan and CT angiography were convincing. He was dischared one week after surgery with full motor power.
pAVFs consist of one or more arterial connections to a single venous channel without any intervening nidus of vessels or capillaries. Intracranial pAVFs differ from arteriovenous malformations (AVMs) owing to the lack of nidus and from dural arteriovenous fistulas in that they derive their arterial supply from pial or cortical arterial vessels. They are rare vascular lesions. In the surgical series of 419 brain AVMs reported by Yasargil, pAVF accounted for 2.4% of the total.29) Little is known regarding the pathophysiological mechanisms giving rise to these lesions. It is possible that a misstep in embryological development of the cerebrovasculature produces these lesions. Alternatively, abnormal angiogenesis and associated vascular growth factors and cytokines may play a role.11)
The abnormality from an AVF arises from its high-flow nature. A communication between an arterial feeder directly into a solitary draining vein without an intervening tangle of vessels creates conditions for rapid high flow. Associated venous varices are produced by the high, turbulent flow from AV shunting.1)2)3)5)8) pAVFs can cause seizures, hemorrhage, headache, neurological deficit, cardiac failure in neonates and infants, symptoms of increased intracranial pressure, and intracranial bruit.6)14)15)25)26)
pAVFs may result from trauma or may be congenital.9)22) Congenital AVFs are usually diagnosed during infancy or early childhood, although many patients reported in the literature are adult. None of our patients had a history of significant head injury.
Natural history of these lesions is unfavorable. Conservative management of pAVFs fistulas has been associated with mortality in 63% of patients.18) Therefore, treatment of these lesions appears to be necessary. Treatment of pial fistulas differs from that of AVMs owing to the lack of a nidus. There are several strategies for treatment of pAVF. Most authors advocated simple disconnection of arteriovenous shunting, either by microsurgery or endovascular embolization.7)10)12)13)17)19)20)21)24)27) This strategy is based on the characteristics of pAVFs, which are high-flow communications between an arterial feeder and a single draining vein without an intervening tangle of vessels. Surgical disconnection of AV shunting offers a higher rate of complete obliteration of fistula than endovascular methods, however, it poses the risks of craniotomy and surgical dissection.11) Endovascular applications have been reported as successful means of disconnecting AV fistulae, particularly in deep and eloquent areas. Some angiogeometric configurations, such as multiple arterial connections or drainage of a normal cortical vein into the venous channel of a pAVF, are unfavorable for endovascular flow disconnection.
Some authors reported treatment of pAVF by surgical ligation of both arterial feeders and draining veins and then removal of the varix.4)16)23) This could be an appropriate option, particularly in lesions with large varices and significant mass effect because it reduces the volume of the lesion better and faster than other methods.
Both of our patients had very large surgically accessible varices that convinced us to perform resection of the lesion. Careful evaluation of angiography was helpful to us in finding vascular connections intraoperatively. In Case 2, there were small feeding arteries that were not obvious on DSA and we think that only surgical microdissection could detect all of them. In both patients, varix had thick walls and was surrounded by arachnoid, which made its dissection from other tissues possible. There was no new neurological deficit and the postoperative course was uneventful. Therefore, surgical ligation and resection of giant varix resulted in successful treatment in our patients.
References
1. Almeida GM, Shibata MK. Hemispheric arteriovenous fistulae with giant venous dilation. Childs Nerv Syst. 1990; 6. 6(4):216–219. PMID: 2383876.

2. Aoki N, Sakai T, Oikawa A. Intracranial arteriovenous fistula manifesting as progressive neurological deterioration in an infant: case report. Neurosurgery. 1991; 4. 28(4):619–622. discussion 622-3. PMID: 2034363.

3. Barnwell SL, Ciricillo SF, Halbach VV, Edwards MS, Cogen PH. Intracerebral arteriovenous fistulas associated with intraparenchymal varix in childhood: case reports. Neurosurgery. 1990; 1. 26(1):122–125. PMID: 2294462.

4. Bendok BR, Getch CC, Frederiksen J, Batjer HH. Resection of a large arteriovenous fistula of the brain using low-flow deep hypothermic cardiopulmonary bypass: technical case report. Neurosurgery. 1999; 4. 44(4):888–890. discussion 890-1. PMID: 10201318.

5. Carrillo R, Carreira LM, Prada J, Rosas C, Egas G. Giant aneurysm arising from a single arteriovenous fistula in a child. Case report. J Neurosurg. 1984; 5. 60(5):1085–1088. PMID: 6716144.
6. Garcia-Monaco R, de Victor D, Mann C, Hannedouche A, Terbrugge K, Lasjaunias P. Congestive cardiac manifestations from cerebrocranial arteriovenous shunts. Endovascular management in 30 children. Childs Nerv Syst. 1991; 2. 7(1):48–52. PMID: 2054809.
7. Garcia-Monaco R, Taylor W, Rodesch G, Alvarez H, Burrows P, Coubes P, et al. Pial arteriovenous fistula in children as presenting manifestation of Rendu-Osler-Weber disease. Neuroradiology. 1995; 1. 37(1):60–64. PMID: 7708192.

8. Giller CA, Batjer HH, Purdy P, Walker B, Mathews D. Interdisciplinary evaluation of cerebral hemodynamics in the treatment of arteriovenous fistulae associated with giant varices. Neurosurgery. 1994; 10. 35(4):778–782. discussion 782-4. PMID: 7808630.

9. Halbach VV, Higashida RT, Hieshima GB, Hardin CW, Dowd CF, Barnwell SL. Transarterial occlusion of solitary intracerebral arteriovenous fistulas. AJNR Am J Neuroradiol. 1989; Jul-Aug. 10(4):747–752. PMID: 2505503.
10. Hermier M, Turjman F, Bozio A, Duquesnel J, Lapras C. Endovascular treatment of an infantile nongalenic cerebral arteriovenous fistula with cyanoacrylate. Childs Nerv Syst. 1995; 8. 11(8):494–498. PMID: 7585691.

11. Hoh BL, Putman CM, Budzik RF, Ogilvy CS. Surgical and endovascular flow disconnection of intracranial pial single-channel arteriovenous fistulae. Neurosurgery. 2001; 12. 49(6):1351–1363. discussion 1363-4. PMID: 11846934.

12. Kikuchi K, Kowada M, Sasajima H. Vascular malformations of the brain in hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber disease). Surg Neurol. 1994; 5. 41(5):374–380. PMID: 8009411.

13. Lee JS, Oh CW, Bang JS, Kwon OK, Hwang G. Intracranial pial arteriovenous fistula presenting with hemorrhage: a case report. J Cerebrovasc Endovasc Neurosurg. 2012; 12. 14(4):305–308. PMID: 23346547.

14. Lownie S, Duckwiler G, Fox A, Drake C. Endovascular therapy of nongalenic cerebral arteriovenous fistulas. In : Viñuela F, Halbach V, Dion J, editors. Interventional neuroradiology: endovascular therapy of the central nervous system. New York: Raven Press;1992. p. 87–106.
15. Lv X, Li Y, Jiang C, Wu Z. Endovascular treatment of brain arteriovenous fistulas. AJNR Am J Neuroradiol. 2009; 4. 30(4):851–856. PMID: 19147710.

16. Meyer FB, Grady RE, Abel MD, Nichols DA, Caminha SS, Robb RA, et al. Resection of a large temporooccipital parenchymal arteriovenous fistula by using deep hypothermic circulatory bypass. Case report. J Neurosurg. 1997; 12. 87(6):934–939. PMID: 9384407.
17. Morimoto T, Yamada T, Hashimoto H, Tokunaga H, Tsunoda S, Sakaki T. Direct approach to intracranial vertebral arteriovenous fistula. Case report. Acta Neurochir (Wien). 1995; 137(1-2):98–101. PMID: 8748878.
18. Nelson K, Nimi Y, Lasjaunias P, Berenstein A. Endovascular embolization of congenital intracranial pial arteriovenous fistulas. Neuroimaging Clin N Am. 1992; 2:309–317.
19. Oh HJ, Yoon SM, Kim SH, Shim JJ. A case of pial arteriovenous fistula with giant venous aneurysm and multiple varices treated with coil embolization. J Korean Neurosurg Soc. 2011; 9. 50(3):248–251. PMID: 22102958.

20. Passacantilli E, Pichierri A, Guidetti G, Santoro A, Delfini R. Surgical treatment of pial cerebellar arteriovenous fistulas with aneurysm of the main feeding artery. Surg Neurol. 2006; 1. 65(1):90–94. PMID: 16378872.

21. Ratliff J, Voorhies RM. Arteriovenous fistula with associated aneurysms coexisting with dural arteriovenous malformation of the anterior inferior falx. Case report and review of the literature. J Neurosurg. 1999; 8. 91(2):303–307. PMID: 10433319.
22. Santosh C, Teasdale E, Molyneux A. Spontaneous closure of an intracranial middle cerebral arteriovenous fistula. Neuroradiology. 1991; 33(1):65–66. PMID: 2027449.

23. Tabatabai SA, Zadeh MZ, Habibi Z, Meybodi AT, Hashemi M. Intracerebral atypical calcification in nongalenic pial arteriovenous fistula: a case report. Cases J. 2008; 11. 1(1):335. PMID: 19019250.

24. Talamonti G, Versari PP, DAliberti G, Villa F, Fontana RA, Collice M. Complex arteriovenous fistula of the brain in an infant Case report. J Neurosurg Sci. 1997; 12. 41(4):337–341. PMID: 9555640.
25. Tomlinson FH, Rufenacht DA, Sundt TM Jr, Nichols DA, Fode NC. Arteriovenous fistulas of the brain and the spinal cord. J Neurosurg. 1993; 7. 79(1):16–27. PMID: 8315463.

26. Vinuela F, Drake CG, Fox AJ, Pelz DM. Giant intracranial varices secondary to high-flow arteriovenous fistulae. J Neurosurg. 1987; 2. 66(2):198–203. PMID: 3806202.
27. Yamashita K, Ohe N, Yoshimura S, Iwama T. Intracranial pial arteriovenous fistula. Neurol Med Chir (Tokyo). 2007; 12. 47(12):550–554. PMID: 18159139.
28. Yang WH, Lu MS, Cheng YK, Wang TC. Pial arteriovenous fistula: a review of literature. Br J Neurosurg. 10. 25(5):580–585. PMID: 21501060.

29. Yasargil MG. Microneurosurgery. Stuttgart: Georg Thieme Verlag;1993.
Fig. 1
(A) Brain computed tomography showing a saccular mass with mural calcification in the right sylvian fissure. (B) Magnetic resonance imaging shows flow void within the lesion. (C) Right carotid angiogram (AP view) shows a giant venous aneurysm fed by one branch of the middle cerebral artery and draining via the vein of Trolard into the superior Sagittal sinus (black arrow). (D) After disconnection of the varix, it was resected totally. (E) Microscopic examination of the resected specimen reveals fragments of hyalinized and calcified vessles with myxoid changes.

Fig. 2
(A) Brain computed tomography showing a hemorrhagic mass in the right parietal area with calcification and dilation of cortical vessels. (B) On lateral view of the right carotid angiogram, multiple branches of the middle cerebral artery are connected directly to the venous system and there is a large venous varix. (C) Intraoperative image shows the varix and associated vessels, which are surrounded by arachnoid. (D) After obliteration of feeding arteries (applied clips are evident) and draining vein, en bloc removal was achieved.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download