Abstract
Objectives
The optimal management of patients with spontaneous intracerebral hemorrhage (ICH) remains controversial. The aim of this study was to evaluate technical results and clinical outcomes of frameless stereotactic aspiration and fibrinolysis using urokinase performed in a single center.
Materials and Methods
The subjects of this study were 62 consecutive patients with spontaneous ICH who were treated with frameless stereotactic aspiration and subsequent fibrinolysis using urokinase between February 2009 and June 2010 in our hospital. The surgical results, procedure-related complications, and clinical outcomes were evaluated.
Results
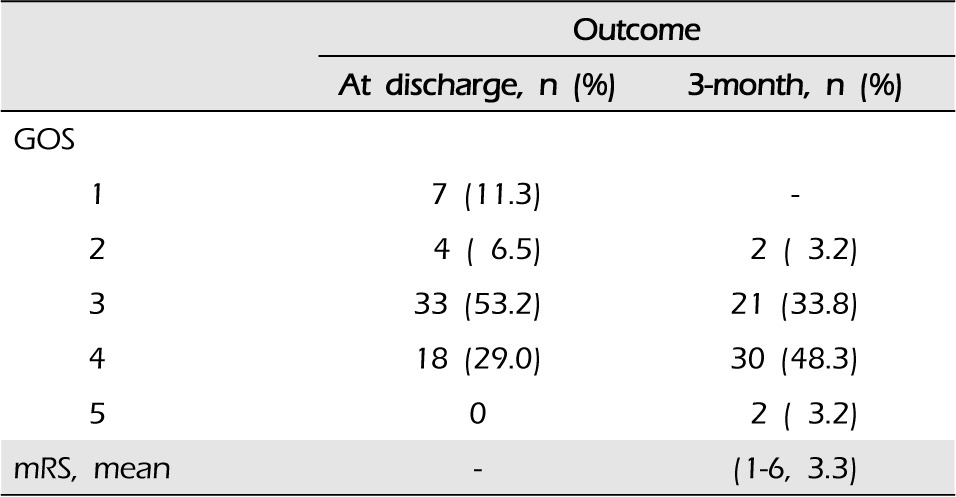
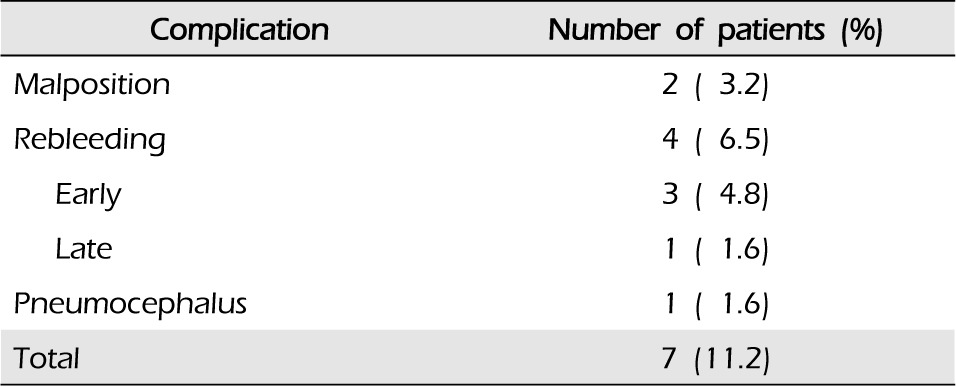
A total of 62 patients were enrolled in the study. The median age was 54 years (range, 32-86). The mean initial Glasgow coma scale score was 7.7 (range 5-11). The mean initial hemorrhage volume was 43 cm3 (range 30-70). Seven patients (11.2%) died of respiratory failure (four patients), postoperative edema (two patients), and heart disease (one patient). There were seven cases of procedure-related complications (11.2%), including malpositioning of catheters (two patients), pneumocephalus (one patient), and rebleeding (four patients, 6.4%). At the three-month follow-up, a good outcome (three-month Glasgow outcome scale > 3) was noted in 32 patients (51.6%).
Spontaneous intracerebral hemorrhage (ICH) comprises 8-30% of all stroke victims. Morbidity and mortality following ICH remain the highest among all forms of cerebral stroke. Traditional medical and surgical approaches, which were mainly developed from clinical experience, can result in only limited neurological improvement in ICH patients.4)8)9)11)13)15)16)18)22)24)25)26)27)33)34)
In recent years, stereotactic aspiration and subsequent fibrinolysis has been developed and accepted as a less invasive and more effective treatment modality for spontaneous ICH owing to the limited damage to overlaid normal brain tissues, compared with conventional surgical removal of ICH. However, the development of stereotactic aspiration of ICH using reliable frameless image guided navigation systems has provided a new and alternative technique.4)5)29)30) Therefore, in this paper, the authors present a single center experience of frameless stereotactic aspiration and subsequent fibrinolysis using urokinase in treatment of spontaneous ICH.
Between February 2009 and June 2010, 62 consecutive patients with spontaneous ICH were treated with frameless stereotactic aspiration and subsequent fibrinolysis in our hospital. There were 18 female patients (29.0%) and 44 male patients (71%) with a mean age of 54 years (range, 32-86 years).
The patient selection criteria for this study were computed tomography based on diagnosis of spontaneous ICH, age > 20 years, hematoma volume ≥ 30 cm3, level of consciousness of Glasgow coma scale (GCS) score > 5 on admission, absence of signs of cerebral herniation, no traumatic or infection related ICH, no suspected underlying clinical or radiological abnormality such as cerebral aneurysm or arteriovenous malformation, no systemic bleeding diathesis and no severe concurrent illness with life expectancy < 6 months, and informed consent from patients or legal representatives.
The diagnosis of spontaneous ICH was made based on the acute onset of neurological symptoms and confirmation by brain computed tomography (CT) scan. Only hemorrhage of putamen or thalamus origin was included.
The baseline level of consciousness was assessed according to the Glasgow coma scale (GCS) and determined at the time of admission during the initial examination. The evaluation of outcomes was made when the patients were discharged from the hospital and during the three-month follow-up. The three-month Glasgow outcome scale (GOS) score was the primary end point. A good outcome was defined as a three-month GOS score > 3. Secondary end points included mortality and modified Rankin scale.
The hematoma volumes were determined by baseline CT scan and 24-hour CT scan performed at bedside in the presence of physicians. Measurement of hematoma volume was attempted without knowledge of each patient's condition. For the bedside length × width × height/2 method, the CT slice with the largest area of hemorrhage was identified. Broderick et al.2) and Kothari et al.14) reported excellent agreement between hematoma volumes measured using the bedside technique and computerized planimetric method.
Following initial resuscitation and radiological investigation, eligible patients underwent repeated CT scan using a planning software protocol that utilized 1 or 2 mm slices, and the obtained images were then loaded onto the station. The goal of placement was to center the catheter along the posterior end of the hemorrhage in order to allow maximum exposure of the blood clot to instilled urokinase. Variables included burr hole placement (usually Kocher's point), the optimal trajectory and the depth to the target according to the shortest depth, and longitudinal axis of hematoma. Frameless stereotactic aspiration was performed using a frameless navigation system (Stryker Navigation System, Kalamazoo, MI, United States). With the integration of a needle and a burr drill following burr-hole placement, it was designed as a hard tunnel. The puncture needle (Silicon catheter, 3.5 mm or 4.0 mm and 15 cm long) was placed in the hemorrhage site in the basal ganglia and mechanical disruption of the blood clot, which means an aspiration of hematoma using a saline-loaded syringe, was manually attempted. A drainage tube was then inserted into the hemorrhage site. After measuring the hemorrhage volume, no further mechanical disruption was attempted due to the concern that aggressive aspiration could precipitate further hemorrhage. A catheter was then connected to a drainage bag. The drainage bag was placed at a level lower than the head to allow natural drainage of hemorrhage. The catheter was placed under the scalp and the scalp was sutured back in place.
The hemorrhage site was first flushed with urokinase 3000 units (diluted with 3 cc of preservative free 0.9% saline) and left for longer than 5 minutes with the catheter closed. The catheter was then reopened using an appropriate method. This procedure was repeated 4-6 times per day for 2-8 days until 80% of the clot was evacuated. The degree of evacuation was confirmed by serial non enhanced brain CT. The first control CT was performed 24 hours after urokinase instillation, subsequent CT was performed by two- or three-day intervals according to the patients' condition. All patients were cared for in the intensive care unit until they became stable enough to be transferred to general wards. Neurological status and vital signs were monitored hourly in the intensive care unit. Hypertension was controlled from the early stage of therapy and the mean arterial blood pressure was maintained between 100 and 120 mmHg by antihypertensive therapy (intravenous nicardipine hydrochloride).
Most patients presented with loss of consciousness (30 patients, 48.4%) and limb weakness (28 patients, 45.2%) on admission. Intraventricular hemorrhage involvement was found in 25 patients (40.3%). The mean initial GCS score was 7.7 (range 5-11). The mean initial hemorrhage volume was 54 cm3 (range 32-86). Hemorrhage aspiration via the inserted catheter was easily achieved in all patients, however, repositioning of the catheter was also necessary in all patients after the initial placement so that it could be placed in the optimal location within the hemorrhage site prior to thrombosis. Initial ICH volume was reduced by an average of 70% (range 40-90%). The average time from symptom onset until the first aspiration was 5 hours (range 2-20). The average period of the needle catheter in situ was 5.2 days (range 2-8 days). The mean frequency of urokinase instillation was 22.5 times (range 12-30).
Perioperative mortality was not reported in this study. Procedure-related complications were reported in seven patients (11.2%) (Table 2). In two cases, catheters were malpositioned and were pulled out a few centimeters. Postsurgical infections secondary to the operation that may require antibiotic therapy were not reported. Pneumocephalus developed in the ventricle after catheter placement and needed to be changed in one patient. This patient did not show any specific symptoms and pneumocephalus was resolved by conservative management. Early rebleeding within the first three postoperative days occurred in three patients and rebleeding after the third postoperative day occurred in one patient. These patients underwent another hematoma puncture and aspiration using new frameless stereotatic aspiration with updated position data in a previously-made burr hole site. The overall rebleeding rate was 6.5% (four patients).
In this study, seven patients (11.2%) died before discharge. Lethal hematoma and frameless stereotatic aspiration following postoperative edema were the cause of death in two patients. Four patients died from bronchopneumonia or sepsis resulting from respiratory failure, and the remaining patient died from heart attack during admission.
At the three-month follow-up, a good outcome (GOS score > 3) was noted in 51.6% of patients (30 patients GOS 4, two patients GOS 5); 21 patients (33.9%) were severely disabled (GOS 3), and two patients (3.2%) remained vegetative. The mean three-month modified Rankin scale score was 3.3 (range 1-6) (Table 1).
Spontaneous ICH is a serious neurosurgical condition requiring intensive care. However, management of patients with spontaneous ICH remains challenging and its optimal treatment option remains controversial owing to the lack of well controlled randomized studies.1)2)3)7)10)12)13)18)21)27)28)32)35) Despite the lack of convincing data, stereotactic removal of hemorrhage and subsequent fibrinolysis at the acute phase has been broadly accepted as an effective treatment option for basal ganglia ICH and a crucial factor for a favorable prognosis because mass effects, perihematomal glutamate level, blood brain barrier permeability, and perihemorrhage edema can be significantly reduced.27)29)33)34)
The use of frameless navigation systems in ICH evacuation was judged to be more effective because it reduced the operation time and postsurgical complications, resulting in a reduction in the need for intensive care, a shorter mean hospital stay, and a reduction of medical cost.4)5)29)30)
A study by Thiex29) reported that frameless stereotactic ICH aspiration was effective in reducing the volume of ICH with comparable clinical outcome and showed an association with targeting an accurate area resulting in less brain damage. In addition, the potential risks of head frame placement, such as an increase in blood pressure secondary to pain could have been avoided by use of frameless stereotactic procedures. An increased blood pressure may increase the risk of hematoma enlargement.
In this study, seven of 62 patients (11.3%) did not survive, however, a good outcome (three-month GOS score > 3) was noted in 51.6 % of patients, which was higher than previously reported results between 20.3% and 55.6% in other studies on frame-based or frameless stereotactic hematoma evacuation for ICH with the figure.4)11)17)20)29) In the study reported by Chen,5) 12 of 48 patients (25%) expired and a good outcome (three-month GOS score > 3) was noted in 41.7% of patients. In published data, the mortality rate of frame-based or frameless stereotactic hematoma evacuation for ICH was reported to be between 20% and 70%.4)5)6)9)17)19)20)29)30)31)34) A study by Thix29) reported a mortality rate after the median long-term follow up of 178 weeks of 38.9% and 25 patients (19.8%) died during the early postoperative period.
In our study, procedure-related complications occurred in seven patients (11.2%), including malpositioning of catheters (two patients), pneumocephalus (one patient), and rebleeding (four patients, 6.5%). Of a total of four cases of rebleeding, three cases were reported during the first three postoperative days and the other case was reported afterwards.
In published data, the prevalence of procedure-related complications, including rebleeding rates, post to frame-based or frameless stereotactic hematoma evacuation for ICH was reported to be between 10% and 39.6%.5)6)29)30) The risk of rebleeding due to intracerebral infusion of a fibrinolytic agent was also reported to be between 7% and 21.9%.23)24)27)29) In the study reported by Chen,5) 14 procedure-related complications (29.2%) were reported, with an overall rebleeding rate of 10.4%. In the study reported by Thix,29) early rebleeding within the first three postoperative days occurred in 11.3% of patients who underwent post to frame-based streotatic surgery and in 9.3% of patients who underwent post to frameless stereotactic surgery. Compared with these study results, the prevalence of procedure-related complications, including the rebleeding rate, was lower in our study. However, due to differences in patient selection criteria and outcome evaluation periods, it was difficult to compare the results of this study with those of existing studies. There are three limitations that need to be addressed regarding the current study. The first limitation is that this study was conducted as a retrospective study with a small sample size and failed to provide a control group, such as patients treated with conservative management or frame-based stereotactic aspiration and subsequent fibrinolysis therapy. The second limitation is that this study failed to reflect other clinical variables that might affect the degree of edema during treatment. The third limitation is that only patients with a small volume of ICH and relatively good GCS score were included in this study, which may not be an accurate reflection of ICH.
This study showed that frameless stereotactic aspiration of spontaneous ICH and subsequent fibrinolytic therapy is a simple, effective, and safe procedure with a lower rebleeding rate and mortality. To establish reliable data on the clinical efficacy of frameless stereotactic aspiration of spontaneous ICH and subsequent fibrinolytic therapy, larger randomized trials, including long-term follow ups, specific inclusion criteria, appropriate medical management, and the optimal time for fibrinolysis therapy will be required in the future.
References
1. Belayev L, Saul I, Curbelo K, Busto R, Belayev A, Zhang Y, et al. Experimental intracerebral hemorrhage in the mouse: Histological, behavioral, and hemodynamic characterization of a double-injection model. Stroke. 2003; 9. 34(9):2221–2227. PMID: 12920264.
2. Broderick JP, Brott T, Tomsick T, Miller R, Huster G. Intracerebral hemorrhage more than twice as common as subarachnoid hemorrhage. J Neurosurg. 1993; 2. 78(2):188–191. PMID: 8421201.

3. Broderick J, Connolly S, Feldmann E, Hanley D, Kase C, Krieger D, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: A guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke. 2007; 6. 38(6):2001–2023. PMID: 17478736.
4. Carhuapoma JR, Barrett RJ, Keyl PM, Hanley DF, Johnson RR. Stereotactic aspiration-thrombolysis of intracerebral hemorrhage and its impact on perihematoma brain edema. Neurocrit Care. 2008; 8(3):322–329. PMID: 18327659.

5. Chen X, Chen W, Ma A, Wu X, Zheng J, Yu X, et al. Frameless stereotactic aspiration and subsequent fibrinolytic therapy for the treatment of spontaneous intracerebral hemorrhage. Br J Neurosurg. 2011; 6. 25(3):369–375. PMID: 20874455.
6. Cho DY, Chen CC, Chang CS, Lee WY, Tso M. Endoscopic surgery for spontaneous basal ganglia hemorrhage: Comparing endoscopic surgery, stereotactic aspiration, and craniotomy in noncomatose patients. Surg Neurol. 2006; 6. 65(6):547–555. discussion 555-6. PMID: 16720167.

7. Cho DY, Chen CC, Lee HC, Lee WY, Lin HL. Glasgow Coma Scale and hematoma volume as criteria for treatment of putaminal and thalamic intracerebral hemorrhage. Surg Neurol. 2008; 12. 70(6):628–633. PMID: 18207500.

8. Elliott J, Smith M. The acute management of intracerebral hemorrhage: A clinical review. Anesth Analg. 2010; 5. 110(5):1419–1427. PMID: 20332192.
9. Fadrus P, Smrcka V, Svoboda T, Maca K, Nadvornik P, Neuman E. Stereotactic evacuation of spontaneous infratentorial hemorrhage with monitoring of intracerebral pressure. Bratisl Lek Listy. 2004; 105(5-6):235–239. PMID: 15535116.
10. Hattori N, Katayama Y, Maya Y, Gatherer A. Impact of stereotactic hematoma evacuation on medical costs during the chronic period in patients with spontaneous putaminal hemorrhage: A randomized study. Surg Neurol. 2006; 5. 65(5):429–435. discussion 435. PMID: 16630899.

11. Hokama M, Tanizaki Y, Mastuo K, Hongo K, Kobayashi S. Indications and limitations for CT-guided streotaxic surgery of hypertensive intracerebral haemorrhage, based on the analysis of postoperative complications and poor ability of daily living in 158 cases. Acta Neurochir (Wien). 1993; 125(1-4):27–33. PMID: 8122552.
12. Hsieh CT, Chen CY, Chiang YH, Chang CH, Chang CF. Role of diffusion tensor imaging in a patient with spontaneous intracerebral hematoma treated by stereotactic evacuation. Surg Neurol. 2008; 7. 70(1):75–78. PMID: 17707485.

13. Itabashi R, Toyoda K, Yasaka M, Kuwashiro T, Nakagaki H, Miyashita F, et al. The impact of hyperacute blood pressure lowering on the early clinical outcome following intracerebral hemorrhage. J Hypertens. 2008; 10. 26(10):2016–2021. PMID: 18806626.

14. Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996; 8. 27(8):1304–1305. PMID: 8711791.

15. Little KM, Alexander MJ. Medical versus surgical therapy for spontaneous intracranial hemorrhage. Neurosurg Clin N Am. 2002; 7. 13(3):339–347. PMID: 12486923.

16. Marquardt G, Wolff R, Janzen RW, Seifert V. Basal ganglia haematomas in non-comatose patients: Subacute stereotactic aspiration improves long-term outcome in comparison to purely medical treatment. Neurosurg Rev. 2005; 1. 28(1):64–69. PMID: 15455261.

17. Marquardt G, Wolff R, Sager A, Janzen RW, Seifert V. Subacute stereotactic aspiration of haematomas within the basal ganglia reduces occurrence of complications in the course of haemorrhagic stroke in non-comatose patients. Cerebrovasc Dis. 2003; 15(4):252–257. PMID: 12686788.

18. Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the international surgical trial in intracerebral haemorrhage (STICH): A randomised trial. Lancet. 2005; Jan-Feb. 365(9457):387–397. PMID: 15680453.

19. Mohadjer M, Braus DF, Myers A, Scheremet R, Krauss JK. CT-stereotactic fibrinolysis of spontaneous intracerebral hematomas. Neurosurg Rev. 1992; 15(2):105–110. PMID: 1635623.

20. Montes JM, Wong JH, Fayad PB, Awad IA. Stereotactic computed tomographic-guided aspiration and thrombolysis of intracerebral hematoma: Protocol and preliminary experience. Stroke. 2000; 4. 31(4):834–840. PMID: 10753984.
21. Morgan T, Awad I, Keyl P, Lane K, Hanley D. Preliminary report of the clot lysis evaluating accelerated resolution of intraventricular hemorrhage (CLEAR-IVH) clinical trial. Acta Neurochir Suppl. 2008; 105:217–220. PMID: 19066112.

22. Naval NS, Nyquist P, Carhuapoma JR. ICH aspiration and thrombolysis. J Neurol Sci. 2007; 10. 261(1-2):80–83. PMID: 17524425.

23. Niizuma H, Shimizu Y, Yonemitsu T, Nakasato N, Suzuki J. Results of stereotactic aspiration in 175 cases of putaminal hemorrhage. Neurosurgery. 1989; 6. 24(6):814–819. PMID: 2664544.

24. Niizuma H, Otsuki T, Johkura H, Nakazato N, Suzuki J. CT-guided stereotactic aspiration of intracerebral hematoma-result of a hematoma-lysis method using urokinase. Appl Neurophysiol. 1985; 48(1-6):427–430. PMID: 3915660.
25. Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001; 5. 344(19):1450–1460. PMID: 11346811.

26. Tan SH, Ng PY, Yeo TT, Wong SH, Ong PL, Venketasubramanian N. Hypertensive basal ganglia hemorrhage: A prospective study comparing surgical and nonsurgical management. Surg Neurol. 2001; 11. 56(5):287–292. discussion 292-3. PMID: 11749988.

27. Teernstra OP, Evers SM, Lodder J, Leffers P, Franke CL, Blaauw G. Multicenter randomized controlled trial (SICHPA). Stereotactic treatment of intracerebral hematoma by means of a plasminogen activator. Stroke. 2003; 4. 34(4):968–974. PMID: 12649510.

28. Thai QA, Pradilla G, Legnani FG, Kretzer RM, Hsu W, Tamargo RJ. Lysis of intracerebral hematoma with stereotactically implanted tissue plasminogen activator polymers in a rabbit model. J Neurosurg. 2006; 9. 105(3):424–429. PMID: 16961138.

29. Thiex R, Rohde V, Rohde I, Mayfrank L, Zeki Z, Thron A, et al. Frame-based and frameless stereotactic hematoma puncture and subsequent fibrinolytic therapy for the treatment of spontaneous intracerebral hemorrhage. J Neurol. 2004; 12. 251(12):1443–1450. PMID: 15645342.

30. Umebayashi D, Mandai A, Osaka Y, Nakahara Y, Tenjin H. Effects and complications of stereotactic aspiration for spontaneous intracerebral hemorrhage. Neurol Med Chir (Tokyo). 2010; 50(7):538–544. PMID: 20671378.

31. Vespa P, McArthur D, Miller C, O'Phelan K, Frazee J, Kidwell C, et al. Frameless stereotactic aspiration and thrombolysis of deep intracerebral hemorrhage is associated with reduction of hemorrhage volume and neurological improvement. Neurocrit Care. 2005; 2(3):274–281. PMID: 16159075.

32. Wagner KR, Xi G, Hua Y, Zuccarello M, de Courten-Myers GM, Broderick JP, et al. Ultra-early clot aspiration after lysis with tissue plasminogen activator in a porcine model of intracerebral hemorrhage: Edema reduction and blood-brain barrier protection. J Neurosurg. 1999; 3. 90(3):491–498. PMID: 10067918.

33. Wu G, Sheng F, Wang L, Wang F. The pathophysiological time window study of performing minimally invasive procedures for the intracerebral hematoma evacuation in rabbit. Brain Res. 2012; 7. 1465:57–65. PMID: 22658751.

34. Zhou H, Zhang Y, Liu L, Han X, Tao Y, Tang Y, et al. A prospective controlled study: Minmally invasive stereotactic puncture therapy versus conventional craniotomy in the treatment of acute intracerebral hemorrhage. BMC Neurol. 2011; 6. 11:76. PMID: 21699716.

35. Zhou H, Zhang Y, Liu L, Huang Y, Tang Y, Su J, et al. Minimally invasive stereotactic puncture and thrombolysis therapy improves long-term outcome after acute intracerebral hemorrhage. J Neurol. 2011; 4. 258(4):661–669. PMID: 21340523.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download