Abstract
With rapidly increasing numbers of neuroendovascular procedures performed annually in recent years, use of arterial closure devices after femoral artery access has been exceedingly common secondary to reduced time to hemostasis, decreased patient discomfort, earlier mobilization, and shortened hospital stay. Although uncommon, use of these devices can lead to a different spectrum of complications, as compared to manual compression. Ischemic symptoms following the use of these devices can have unexpected clinical sequelae and can occur in a delayed fashion. Awareness and recognition of such complications is important with the dramatically increased use of these devices in recent years. We report on a case of delayed vascular complication manifesting as vascular claudication following use of the AngioSeal closure device.
Neurointerventional surgery has shown exponential growth in the last decade secondary to development of newer devices and more sophisticated techniques enabling neurointerventional surgeons to treat a broader and more complex spectrum of pathologies with increased success rates and safety profile. Femoral artery arteriotomy remains the preferred method of vascular access for both diagnostic and interventional procedures in the United States and the rest of the world.6) While manual compression has historically been regarded as the "gold standard" for achievement of vascular closure after femoral artery puncture, it requires immobilization for up to 6 hours or so after the procedure and can often be associated with patient discomfort.
Various vascular closure devices have been developed in recent years in an effort to facilitate more rapid hemostasis, lessen patient discomfort, and shorten post-procedure immobilization.4) While there are data demonstrating the safety and efficacy of these newer devices, there is also an unique set of rare but potentially serious vascular complications associated with these devices, which all neurointerventional surgeons need to be aware of. We report on a rare case of delayed vascular claudication after use of the AngioSeal arteriotomy closure device with a review of pertinent literature.
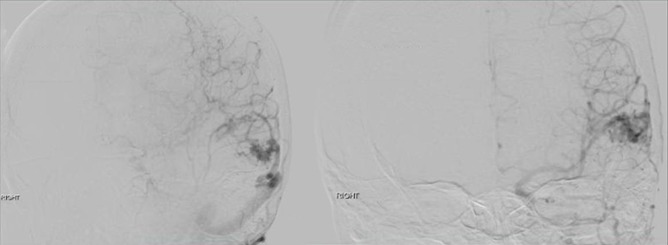
A 26-year-old female presented with worsening headaches, dizziness, and weakness for three months. Magnetic resonance imaging (MRI) of the brain showed the presence of a left parietal lesion concerning for a vascular malformation (Fig. 1). A diagnostic cerebral angiogram was performed to confirm the diagnosis and further characterize the lesion for treatment planning. The right common femoral artery was selectively catheterized using a 5F sheath and a 5F hockey-stick diagnostic angiocatheter with a 0.035 Glidewire were used for selective catheterization of the left internal carotid artery. Cerebral angiogram in anteroposterior/lateral and multiple oblique projections showed a superficial left parietotemporal Spetzler-Martin Grade I arteriovenous malformation (AVM) measuring 15.3×10.3 mm in size (Fig. 2). Then, A 6F AngioSeal closure device (ACD) was deployed successfully at end of the procedure.
Postoperatively, the patient had a small puncture site hematoma with good distal pedal pulses.
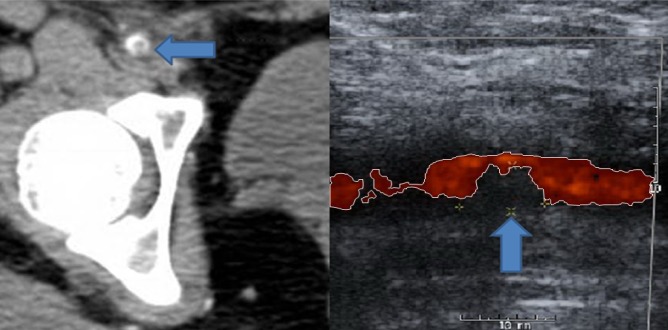
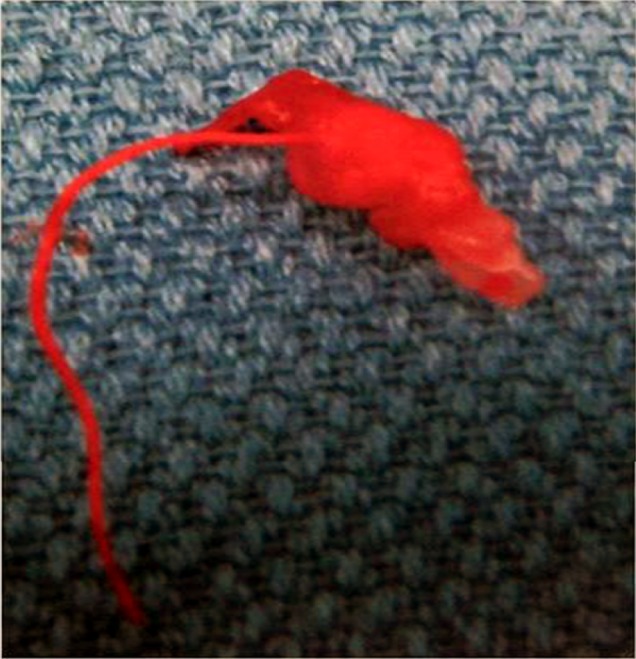
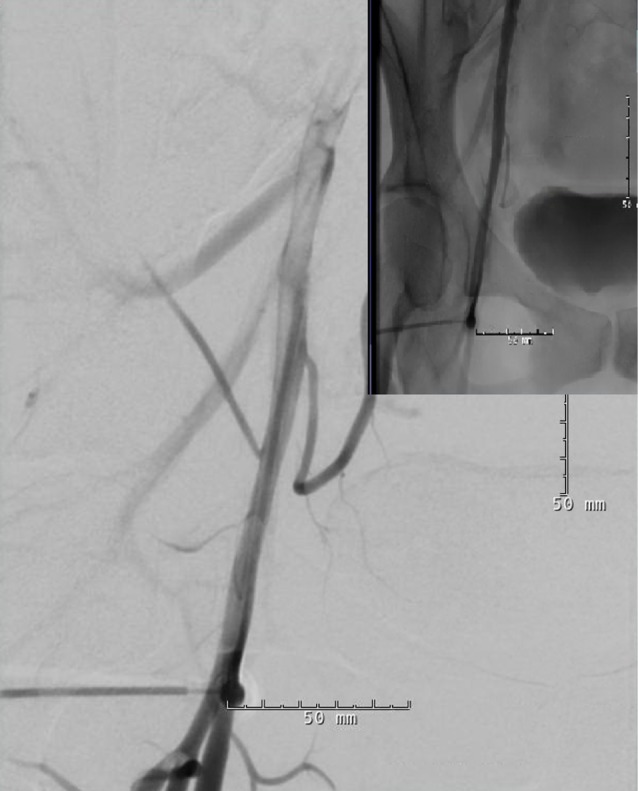
The small groin hematoma appeared stable after a period of observation and she was discharged home from recovery three hours later on the same day of the procedure. However, three days after the procedure, she developed persistent right groin pain with clinical signs of vascular claudication in the right leg manifested by calf pain exacerbated by ambulation. Duplex ultrasound and computed tomography angiography (CTA) of the right femoral artery showed a partially occlusive thrombus in the right common femoral artery (CFA) (Fig. 3). Vascular surgery was consulted and the patient was taken to the operating room in order to undergo thrombectomy. Intraoperatively, it was found that the previously noted "thrombus" was actually the AngioSeal used to close the arteriotomy (Fig. 4). The patient had a stable post-operative course and was discharged in stable condition the day after explanting the AngioSeal.
With rapidly increasing numbers of neuroendovascular procedures performed annually in recent years, technical considerations and complications of peripheral artery access are becoming areas of increasing concern. After femoral artery puncture, adequate hemostasis must be maintained upon removal of the catheter from the artery. Classically, this has been achieved with manual compression with very good safety and efficacy. However, this method requires longer surgeon attention, prolonged periods of immobilization for the patient and thus longer hospital stays and monitoring, which translates into increased hospital costs.10) In addition, this technique is associated with increased patient discomfort and is more difficult with larger arteriotomies.4) In an effort to obviate these limitations, various closure devices have been introduced in clinical practice.
Nevertheless, in some studies questioning the value of routine use of vascular closure devices, the safety of early ambulation (after 2 hours) after the release of manual compression for patients who underwent a diagnostic or therapeutic neuroendovascular procedure without the use of a closure device has been demonstrated.8)
Early ambulation should not be the only concern per se; however, the majority of inpatients who undergo endovascular interventions have vascular access with larger sheaths and are often on antiplatelet medications, therefore, they are likely to be monitored in the hospital overnight and would not be discharged home in few hours anyway.
The advantage of using vascular closure devices under these circumstances are achievement of more rapid hemostasis and vascular closure while reducing patient discomfort and time required for the surgeon's immediate attention. Nevertheless, in high volume centers performing a number of neuroendovascular procedures, arterial puncture closure devices have been developed and used on a routine basis, including the AngioSeal, which is designed to compress the arteriotomy site between a bio-absorbable intravascular foot place and an external collagen sponge in order to circumvent some of the disadvantages associated with manual compression.10) These devices have grown in popularity due to faster rates of hemostasis and avoidance of manual compression. In a recent prospective comparison of neurointerventional treatment via the femoral artery using AngioSeal vs. manual compression, AngioSeal was found to achieve significantly faster hemostasis, with a mean hemostasis of 2.4 +/- 11.8 minutes compared to 44.7 +/- 27.4 minutes in the manual compression group.10) Similarly, results of a meta-analysis on complications of peripheral vascular access during cardiac angiography showed that time to hemostasis was significantly reduced with arterial closure devices compared to manual compression, with a mean difference of 17 minutes; the duration of bed rest was also shorter with arteriotomy closure devices by a mean difference of 10.8 hours.4) However, complication rates of groin hematoma, bleeding, arteriovenous fistula, and pseudoaneurysms have not been shown to be reduced with the use of arteriotomy closure devices. In fact, some studies even suggested a higher rate of complications with arteriotomy closure devices.4)
Vascular complications with arterial closure devices are usually associated with pre-existing peripheral vascular disease, concomitant anticoagulation, and presence of an indwelling sheath for an extended period of time.3) However, the majority of these complications are considered minor and require no further surgical intervention or hospital admission.3)4)10) Meta-analysis comparing the use of vascular closure devices to manual compression demonstrated a relative risk of minor complication rates such as groin hematomas, groin bleeding, and pseudoaneurysm of 1.14, 1.48, and 1.19, respectively.4) In another study of 698 AngioSeal closures conducted at a single institution, the minor complication rate was 2.4%, while the major complication rate was 1.4%. Major complications were defined as those requiring therapy of hospitalization > 48 hours, major therapy, unplanned increase in level of care, or those resulting in permanent adverse sequelae.3) Vascular occlusion is a feared rare complication that is unique to use of vascular closure devices. In the literature, the majority of complications occur on the day of the procedure; however, major complications such as vessel occlusion and stenosis can also occur in delayed fashion necessitating a high index of suspicion for claudication symptoms and close follow-up after the procedure.9) In their retrospective study of femoral artery closures, Carey, et al. suggested that AngioSeal in particular, has a higher rate of occlusion complications, with a rate of 0.7%, compared to other devices.1) One case series by Wille et al. reported six surgically-treated complications associated with AngioSeal, in which five patients had obstructive complications.9) In all of these obstructive cases, the AngioSeal had caused the femoral artery occlusion and the patients required wound exploration, removal of the Angioseal, and endarterectomy with venous patch reconstruction. Four of the five patients had leg ischemia due to occlusion of ipsilateral CFA and superficial femoral artery (SFA). Two of the five patients had a very proximal femoral bifurcation and the puncture site with Angioseal was at the CFA-SFA transition. One had a smaller CFA diameter (4-5 mm) and in another case the vessel occlusion was caused by posterior wall plaque dissection, which was captured by the Angioseal anchor. It seems that in cases where the anchor is absorbed into the vessel lumen, a fibrotic reaction ensues, resulting in occlusion of the artery with a delayed presentation of worsening claudication.9)
Vascular complications associated with arterial closure devices are uncommon but are well documented in the literature.1)4)5)6)7)9)10) Fortunately, major complications requiring surgical intervention and vascular repair are much less common, and when serious complications occur, they usually occur acutely in the immediate post-operative period, allowing prompt recognition and management.9) However, the timing of major complications varies in different studies and has often been documented after 24 hours of using arterial closure devices,2) meaning after the patient has already been discharged home, as happened in our case. Plausible risk factors for such complications include age > 70, female, peripheral vascular disease, large size sheath, and arterial puncture very close to the bifurcation, as seen in our case (Fig. 5).6)9) This may potentially impair the proper deployment of the AngioSeal device. In addition, in patients with a puncture close to the bifurcation, it may be prudent to obtain post-operative Duplex ultrasound to ensure proper deployment of the AngioSeal and there should be a low threshold for investigating signs of possible delayed vascular complication in these patients.
While large clinical studies have demonstrated the safety and effectiveness of vascular closure devices, rare but serious complications such as vascular stenosis and occlusion can occur in both acute and delayed fashion. Awareness of these rare complications associated with vascular closure devices, particularly those occurring in a delayed setting outside of direct observation by healthcare professionals, is important for both neurointerventional surgeons and patients. Appropriate pre-procedure counseling of patients receiving these closure devices regarding potential complications is essential in order to educate patients to recognize signs and symptoms of these rare complications and to minimize permanent sequelae.
References
1. Carey D, Martin JR, Moore CA, Valentine MC, Nygaard TW. Complications of femoral artery closure devices. Catheter Cardiovasc Interv. 2001; 1. 52(1):3–7. discussion 8. PMID: 11146512.

2. Chung JJ, Yu JS, Kim JH, Nam SJ, Kim MJ. Intraabdominal complications secondary to ventriculoperitoneal shunts: CT findings and review of the literature. AJR Am J Roentgenol. 2009; 11. 193(5):1311–1317. PMID: 19843747.

3. Geyik S, Yavuz K, Akgoz A, Koc O, Peynircioglu B, Cil B, et al. The safety and efficacy of the Angio-Seal closure device in diagnostic and interventional neuroangiography setting: a single-center experience with 1,443 closures. Neuroradiology. 2007; 9. 49(9):739–746. PMID: 17594084.

4. Koreny M, Riedmüller E, Nikfardjam M, Siostrzonek P, Müllner M. Arterial puncture closing devices compared with standard manual compression after cardiac catheterization: systematic review and meta-analysis. JAMA. 2004; 1. 291(3):350–357. PMID: 14734598.

5. Nikolsky E, Mehran R, Halkin A, Aymong ED, Mintz GS, Lasic Z, et al. Vascular complications associated with arteriotomy closure devices in patients undergoing percutaneous coronary procedures: a meta-analysis. J Am Coll Cardiol. 2004; 9. 15. 44(6):1200–1209. PMID: 15364320.

6. Patel MR, Jneid H, Derdeyn CP, Klein LW, Levine GN, Lookstein RA, et al. Arteriotomy closure devices for cardiovascular procedures: a scientific statement from the American Heart Association. Circulation. 2010; 11. 122(18):1882–1893. PMID: 20921445.
7. Vaitkus PT. A meta-analysis of percutaneous vascular closure devices after diagnostic catheterization and percutaneous coronary intervention. J Invasive Cardiol. 2004; 5. 16(5):243–246. PMID: 15152128.
8. Wagenbach A, Saladino A, Daugherty WP, Cloft HJ, Kallmes DF, Lanzino G. Safety of early ambulation after diagnostic and therapeutic neuroendovascular procedures without use of closure devices. Neurosurgery. 2010; 3. 66(3):493–496. discussion 496-7. PMID: 20124936.

9. Wille J, Vos JA, Overtoom TT, Suttorp MJ, van de Pavoordt ED, de Vries JP. Acute leg ischemia: the dark side of a percutaneous femoral artery closure device. Ann Vasc Surg. 2006; 3. 20(2):278–281. PMID: 16550481.

10. Wong HF, Lee CW, Chen YL, Wu YM, Weng HH, Wang YH, et al. Prospective comparison of angio-seal versus manual compression for hemostasis after neurointerventional procedures under systemic heparinization. AJNR Am J Neuroradiol. 2013; 2. 34(2):397–401. PMID: 22859279.

Fig. 1
Axial T1 and T2 weighted Magnetic resonance imaging (MRI) of the brain showing the presence of flow voids in the left parietal lobe suggestive of a vascular malformation.
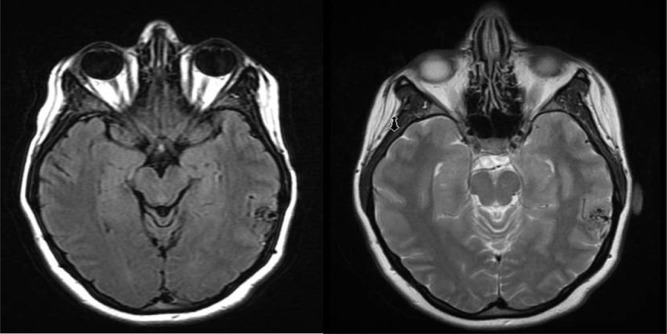
Fig. 2
Lateral and AP views of the left internal carotid artery injection during cerebral angiography showing a superficial left parietotemporal arteriovenous malformation (AVM) measuring 15.3×10.3 mm in size.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download