Abstract
Objective
The aim of this study was to document the natural course of asymptomatic adult moyamoya disease (MMD) and the factors related to disease progression to aid in treatment decisions.
Materials and Methods
Among 459 adult MMD patients (aged ≥ 20 years), 42 patients were included in this retrospective cohort study. Clinical records of adult asymptomatic MMD patients (n = 42) and follow-up data from September 2013 were reviewed to determine the factors related to disease progression.
Results
The mean age of patients at the time of diagnosis was 41.2 years (range, 23-64 years), and the mean follow-up period was 37.3 months (range, 7.4-108.7 months). Of the 42 patients and 75 hemispheres, there were 12 patients (28.6%) and 13 hemispheres (17.3%) with disease progression. There were four hemispheres (5.3%) with symptomatic progression (three hemorrhage, one transient ischemic attack) and nine hemispheres (12.0%) with asymptomatic radiographic progression. There were no relationships with sex, diabetes, hypertension, thyroid disease, family history of MMD, or family history of stroke. However, reduced initial cerebrovascular reserve capacity was observed in seven hemispheres (9.3%) in patients with disease progression. A relationship was found between disease progression and initial cerebrovascular reserve capacity (p = 0.05). None of the patients underwent bypass surgery during the follow-up period.
Moyamoya disease (MMD), characterized by progressive stenosis or occlusion of the bilateral intracranial internal carotid arteries, is associated with abnormal vascular networks at the base of the brain, termed the "moyamoya" vessels.13)15) "Probable MMD" is characterized by unilateral hemispheric involvement and exclusion of the following basic diseases (arteriosclerosis, autoimmune disease, meningitis, brain neoplasm, down syndrome, Recklinghausen's disease, head trauma, irradiation to the head.1) MMD generally peaks at two different ages, the second and fifth decades.8) A number of studies have reported that surgical revascularization for symptomatic MMD is effective in pediatric patients.2)12)14) Owing to disease progression, early surgical interventions in pediatric patients can reduce morbidity resulting from stroke even in the absence of symptoms.7)10) However, evidence regarding the incidence and natural course of asymptomatic MMD in adults is limited.
The prevalence of asymptomatic adult MMD has recently increased due to development of noninvasive diagnostic techniques and the increase in regular medical checkups.11) Given the lack of guidance regarding treatment in these patients, the aim of this study was to document the natural course of asymptomatic adult MMD to aid in treatment decisions.
We reviewed data from May 2003 to December 2012 in the clinical database of our institute for identification of patients who were diagnosed with MMD or probable MMD according to published guidelines.1) We included probable MMD in this study because we think that MMD and probable MMD are inseparable diseases. Of the 587 MMD patients identified, 459 patients were adults (aged ≥ 20 years) at the time of diagnosis. Patients were considered to have asymptomatic MMD when it was detected without the presence of an ischemic event, a hemorrhagic event, or a focal neurological deficit. As a result, 42 patients were included in this retrospective cohort study. Clinical records were reviewed retrospectively, and follow-up data from September 2013 were evaluated.
All of the patients had complained of a simple headache and underwent subsequent brain magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA). Diagnosis of MMD was based on MRI and MRA findings, and 21 patients underwent conventional angiography to confirm the diagnosis. Cerebral perfusion was measured in 41 patients using 99mTc-hexamethylpropylene amine oxime single photon emission computed tomography (SPECT), xenon computed tomography (CT), or positron emission tomography (PET), and cerebral reserve capacity was assessed after an intravenous injection of acetazolamide. Cerebral perfusion and reserve capacity images were interpreted as normal or decreased status, respectively, by the same competent professor of nuclear medicine.
Analysis of the relationships between disease progression and associated factors was performed using χ2 tests. The progression free survival rate was estimated by Kaplan-Meier analysis. The significance of the differences was assessed using the log-rank test. IBM SPSS V. 21 (IBM Corp., Armonk, NY, United States) was used in performance of statistical analyses.
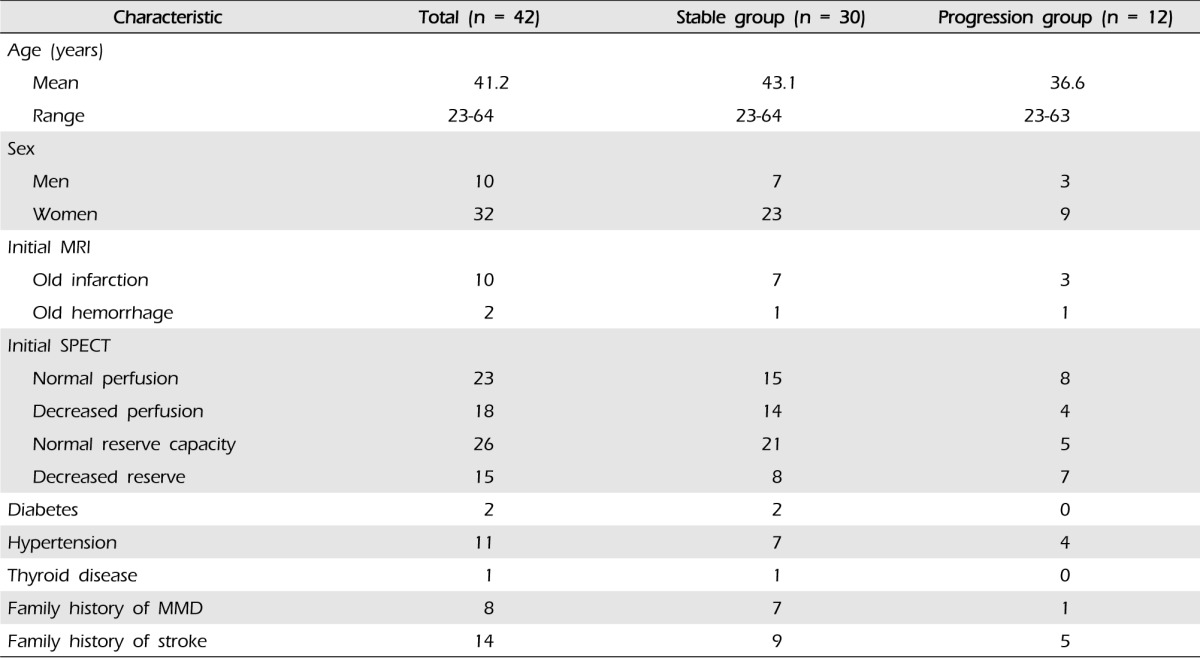
Of the 42 patients identified with asymptomatic adult MMD, 33 patients had bilateral MMD, and nine patients had probable MMD, resulting in inclusion of 75 hemispheres. The sample included 32 (76.2%) women and 10 (23.8%) men (Table 1). The mean age at the time of diagnosis was 41.2 years (range, 23-64 years). The mean follow-up period was 37.3 months (range, 7.4-108.7 months), during which time none of the patients underwent bypass surgery. Additional patient characteristics are shown in Table 1.
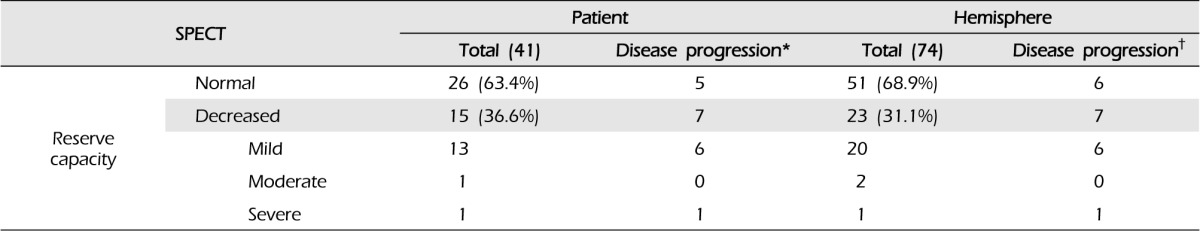
According to the initial MRIs, 12 patients (28.6%) had old lacunar infarcts or microbleeding without symptoms. Reduced cerebrovascular reserve capacity (CVRC) was detected in the initial SPECT in 15 patients (23 hemispheres, 30.7%) (Table 2). Disease progression was divided according to two categories: symptomatic progression, in which any neurological symptom developed, and asymptomatic radiographic progression, in which abnormal findings were detected in a follow-up radiological evaluation. Symptomatic is frequently referred to as "clinical progression" in other reports.11)
Of the 42 patients and 75 hemispheres, there were 12 patients (28.6%) and 13 hemispheres (17.3%) with either category of disease progression. There were no relationships with sex, diabetes, hypertension, thyroid disease, family history of MMD, or family history of stroke. However, the initial CVRC was reduced in six patients (14.3%) and seven hemispheres (9.3%) in the patients with disease progression (Table 2).
Symptomatic progression (hemorrhagic or ischemic stroke) occurred in four patients, all women. The mean ± SD duration from MMD diagnosis to event occurrence was 20.8 ± 15.7 months (range, 7.6-41.1 months). Reduced CVRC was observed in the initial SPECT for all four patients. Three patients experienced hemorrhagic stroke and basal ganglia intracerebral hemorrhage. Two patients underwent decompressive craniectomy and removal of a hematoma due to a large amount of hemorrhage. The other patient received conservative treatment. The fourth patient experienced a transient ischemic attack after cesarean delivery, followed by intermittent occurrence of symptoms. Therefore, we recommended bypass surgery, but the patient did not make a decision.
Eight patients (nine hemispheres, 12.0%) showed asymptomatic radiographic progression during the follow-up period. In six patients, the follow-up SPECT showed reduced CVRC compared with the initial SPECT. A silent cerebral infarction on the left corona radiate was detected in one patient, and the follow-up MRI showed focal microbleeding at the left cingulate gyrus, without symptoms, in the final patient.
Of the 42 patients included in this study, MMD progression was evident in 12 patients (13 of 75 hemispheres), representing almost a third of the sample. Symptomatic progression, associated with cerebrovascular events, occurred in four of these patients. Three of the four patients suffered an intracerebral hemorrhage, while the fourth experienced a transient ischemic stroke.
In general, child MMD is considered to be progressive while adult MMD is relatively stable.15) However, the current results and those of a previous study that reported disease progression in 15 of 120 adult patients (12.5%) suggest otherwise.9) Further, higher rate of hemorrhagic stroke in adult MMD than that in children has been well documented.3)13) There are differences in disease presentation and features between adults and children with moyamoya.13) In addition, in children, disease diagnosis can be delayed and symptoms classified inaccurately owing to an inability to communicate.5)
Suzuki and Takaku proposed an angiographic staging system for MMD.15) However, the stages are not correlated with disease severity. Instead, CVRC has been known to be more effective for monitoring the progression of the disease; the patients are often in a chronic state of cerebral vasodilation, and maintenance of cerebral blood flow is necessary.4) Therefore, we evaluated the CVRC of the patients. Despite a lack of differences in the majority of factors between patients with and without disease progression, a relationship was observed with CVRC; decreased CVRC was evident with more progressed disease (Table 2). This was supported by the results of the Kaplan-Meier cumulative event-free survival analysis, in which a significant difference was observed between patients with progressive disease and a reduced initial CVRC and those with progressive disease and a normal initial CVRC (Fig. 1). This difference was also evident in patients with symptomatic progression (Fig. 2). Based on these results, CVRC may be an important indicator for patients with asymptomatic MMD, and closer observation or early revascularization surgery may be necessary in patients with reduced CVRC.
In our institute, symptomatic progression is an important indication for revascularization surgery in MMD patients. However, in this study, none of the patients underwent surgery during the follow-up period. Only one patient for whom an operation was recommended did not make a decision regarding surgery. Therefore, we could not assess disease progression following revascularization surgery. However, another study conducted with patients with asymptomatic MMD reported that disease progression did not occur after revascularization surgery.9) This is an interesting finding for this treatment modality; early revascularization surgery may be considered for asymptomatic MMD patients who had initial decreased CVRC.
This study is one of only a few studies conducted with adult patients with asymptomatic MMD. A multicenter survey conducted in 2007 included 40 patients with asymptomatic MMD; however, patients were all older adults.9) In a study investigating 40 patients (74 hemispheres) for a median follow-up period of 32 months, Jo et al. also reported that asymptomatic MMD in adults is not a stable disease. However, in this report, stroke rate was 0%, but in our study, three patients (3/42, 7.1%) had stroke during the follow-up period.6)
This study has certain limitations. A retrospective review of medical records was used for collection of patient data. In addition, the sample size was small; however, MMD itself, particularly in the asymptomatic state, is very rare. Therefore, we plan to conduct future multi-center studies to guide treatment in adult, asymptomatic adult MMD patients.
The aim of this study was to document the disease course and factors related to disease progression in adults with asymptomatic MMD. Based on our results and those of previous studies, we conclude that asymptomatic adult MMD can no longer be considered a permanent stable disease. Therefore, close observation with regular brain imaging study is required for asymptomatic adult MMD patients, particularly patients with reduced CVRC.
References
1. Fukui M. Guidelines for the diagnosis and treatment of spontaneous occlusion of the circle of Willis ('moyamoya' disease). Research committee on spontaneous occlusion of the circle of Willis (Moyamoya disease) of the Ministry of Health and Welfare, Japan. Clin Neurol Neurosurg. 1997; 10. 99(Suppl 2):S238–S240. PMID: 9409446.
2. Fung LW, Thompson D, Ganesan V. Revascularisation surgery for paediatric moyamoya: a review of the literature. Childs Nerv Syst. 2005; 5. 21(5):358–364. PMID: 15696334.

3. Han DH, Nam DH, Oh CW. Moyamoya disease in adults: characteristics of clinical presentation and outcome after encephalo-duro-arterio-synangiosis. Clin Neurol Neurosurg. 1997; 10. 99(Suppl 2):S151–S155. PMID: 9409427.
4. Horie N, Morikawa M, Nozaki A, Hayashi K, Suyama K, Nagata I. "Brush Sign" on susceptibility-weighted MR imaging indicates the severity of moyamoya disease. AJNR Am J Neuroradiol. 2011; 10. 32(9):1697–1702. PMID: 21799039.

5. Jea A, Smith ER, Robertson R, Scott RM. Moyamoya syndrome associated with Down syndrome: outcome after surgical revascularization. Pediatrics. 2005; 11. 116(5):e694–e701. PMID: 16263984.

6. Jo KI, Yeon JY, Hong SC, Kim JS. Clinical course of asymptomatic adult moyamoya disease. Cerebrovasc Dis. 2014; 37(2):94–101. PMID: 24435045.

7. Kim SK, Seol HJ, Cho BK, Hwang YS, Lee DS, Wang KC. Moyamoya disease among young patients: its aggressive clinical course and the role of active surgical treatment. Neurosurgery. 2004; 4. 54(4):840–844. discussion 844-6. PMID: 15046649.

8. Kuriyama S, Kusaka Y, Fujimura M, Wakai K, Tamakoshi A, Hashimoto S, et al. Prevalence and clinicoepidemiological features of moyamoya disease in Japan: findings from a nationwide epidemiological survey. Stroke. 2008; 1. 39(1):42–47. PMID: 18048855.
9. Kuroda S, Hashimoto N, Yoshimoto T, Iwasaki Y. Research committee on moyamoya disease in Japan. Radiological findings, clinical course, and outcome in asymptomatic moyamoya disease: results of multicenter survey in Japan. Stroke. 2007; 5. 38(5):1430–1435. PMID: 17395863.
10. Lin N, Baird L, Koss M, Kopecky KE, Gone E, Ullrich NJ, et al. Discovery of asymptomatic moyamoya arteriopathy in pediatric syndromic populations: radiographic and clinical progression. Neurosurg Focus. 2011; 12. 31(6):E6.

11. Nanba R, Kuroda S, Takeda M, Shichinohe H, Nakayama N, Ishikawa T, et al. [Clinical features and outcomes of 10 asymptomatic adult patients with moyamoya disease]. No Shinkei Geka. 2003; 12. 31(12):1291–1295. Japanese. PMID: 14719442.
12. Roach ES, Golomb MR, Adams R, Biller J, Daniels S, Deveber G, et al. Management of stroke in infants and children: a scientific statement from a special writing group of the American Heart Association Stroke Council and the Council on cardiovascular disease in the young. Stroke. 2008; 9. 39(9):2644–2691. PMID: 18635845.
13. Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. N Engl J Med. 2009; 3. 360(12):1226–1237. PMID: 19297575.

14. Scott RM, Smith JL, Robertson RL, Madsen JR, Soriano SG, Rockoff MA. Long-term outcome in children with moyamoya syndrome after cranial revascularization by pial synangiosis. J Neurosurg. 2004; 2. 100(2 Suppl Pediatrics):142–149. PMID: 14758941.

15. Suzuki J, Takaku A. Cerebrovascular "moyamoya" disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. 1969; 3. 20(3):288–299. PMID: 5775283.
Fig. 1
Cumulative event free survival rate in patients with asymptomatic moyamoya disease who experienced asymptomatic or symptomatic disease progression (n = 12) CVR = cerebrovascular reserve
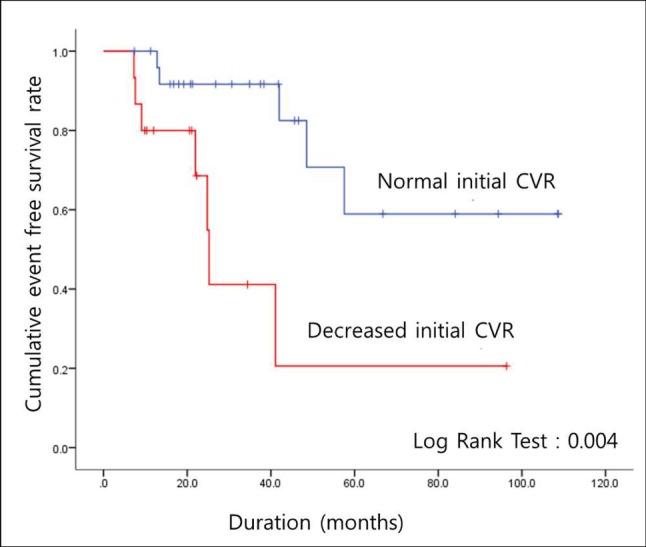
Fig. 2
Cumulative event free survival rate in patients with asymptomatic moyamoya disease who experienced symptomatic disease progression (n = 4) CVR = cerebrovascular reserve





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download