Abstract
Objective
The clinico-radiologic features of the spontaneous basal ganglia hemorrhage (BGH) may often differ one from another, according to its regional location. Therefore, we attempted to classify the BGH into regional subgroups, and to extrapolate the distinct characteristics of each group of BGH.
Materials and Methods
A total of 103 BGHs were analyzed by retrospective review of medical records. BGH was classified according to four subgroups; anterior BGH; posterior BGH; lateral BGH; massive BGH.
Results
The most common BGH was the posterior BGH (56, 54.4%), followed by the lateral BGH (26, 25.2%), the massive BGH (12, 11.7%), and the anterior BGH (9, 8.7%). The shape of hemorrhage tended to be round in anterior, irregular in posterior, and ovoid in lateral BGH. A layered density of hematoma on initial computed tomography showed correlation with hematoma expansion (p = 0.016), which was observed more often in the postero-lateral group of BGH than in the anterior BGH group. Relatively better recovery from the initial insult was observed in the lateral BGH group than in the other regional BGH groups. The proportion of poor outcome (modified Rankin scale 4, 5, 6) was 100% in the massive, 41.1% in the posterior, 34.6% in the lateral, and 0% in the anterior BGH group.
Spontaneous basal ganglia hemorrhage (BGH) is the most common hemorrhagic stroke, imposing significant socio-economic burden on the victims.18) Anatomically, the basal ganglia generally refer to a set of subcortical nuclei, such as striatum, composed of the caudate nucleus and putamen, globus pallidus, substantia nigra, and subthalamic nucleus. The ventricles and internal capsule are adjacent to the basal ganglia. The basal ganglia are supplied by various deep perforators.7)11) Functionally, the basal ganglia play a crucial role in neurologic pathways, including motor, sensory, and cognition.14) Therefore, BGH may often present with a broad spectrum of clinical manifestations, radiologic features, and outcomes according to its regional location.
In this study, we attempted to classify BGHs into regional subgroups according to anatomic location, and to extrapolate the distinct characteristics of each group of BGH, which may affect clinical presentation, course, and prognosis.
A total of 247 patients diagnosed with spontaneous intracerebral hemorrhage from January 2008 to December 2012 at the author's institution were reviewed retrospectively. Patients with an underlying cause such as trauma, vascular disease, tumor, etc. were excluded from this study. As a result, 103 patients met the criteria of the spontaneous BGH group. Patients' data were collected by review of medical records and imaging studies. Functional status was assessed by modified Rankin scale (mRS) at the time of admission, discharge and six months after discharge, and was categorized according to good (0-3) or poor (4-6) group. Motor weakness was estimated according to manual muscle strength scale and the worst affected site was recorded. Motor weakness was dichotomized according to good (4-5) or poor (0-3).
BGHs were classified into four subgroups according to anatomical region based on computed tomography (CT) scans; the anterior BGH mainly involves the caudate head, and possibly extends to the anterior limb of the internal capsule and the rostral part of the putamen; the posterior BGH refers to hemorrhage mainly involving putamen, globus pallidus, and posterior limb of the internal capsule; the lateral BGH is located below the insular cortex and outside the putamen; the massive BGH occupies all three other regions.
Hematoma volume was measured using the ABC/2 method on non-contrast brain CT scan.10) Hematoma expansion was defined as a minimum volume increment of 30% documented on follow-up CT scan within 48 hours compared to initial CT scan.9)
Chi-square test, Fisher exact test, and Kruskal-Wallis test were used for analysis of data. Risk factors contributing to poor outcome (mRS 4-6 at six months) were determined by single and multiple logistic regression analysis. IBM SPSS Statistics software version 20.0.0 (IBM, Armonk, NY, United States) was used for all statistical analyses. Multiple comparisons were performed using pairwise Mann-Whitney test with Bonferroni correction. p value < 0.05 was considered statistically significant. Correlation between double layer on initial CT and hematoma expansion was calculated using Pearson's correlation equation.
No significant differences with regard to age, sex, hematoma expansion, anti-platelet medication and history of diabetes, hypertension, smoking and alcohol consumption were observed between the four subgroups. Patients' demographic characteristics are shown in Table 1.
The largest mean amount of hematoma was observed in massive type, and the smallest volume was observed in anterior type (p = 0.005), while posterior and lateral BGHs were similarly intermediate in amount. Initial GCS score was the highest in anterior type, followed by posterior, lateral, and lowest in massive type.
Layered density on initial CT was noted in 45 BGHs, of which 13 BGHs (28.9%) had expanded on short-term interval follow-up CT. On the correlation test, double layer showed strong correlation with further hematoma expansion (p = 0.016, coefficient = 0.237).
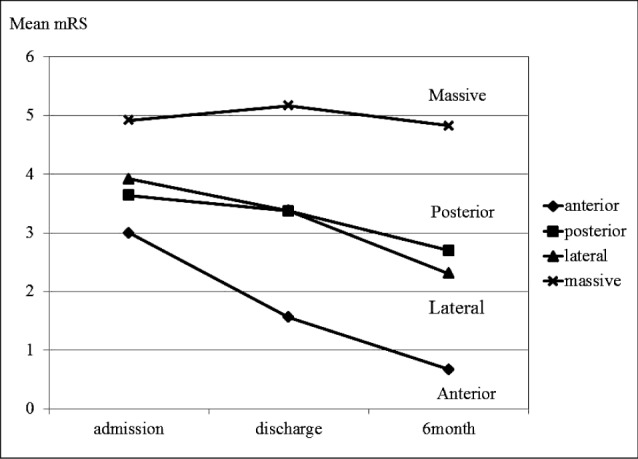
On follow up check the best functional outcome was observed in anterior type (mean mRS = 0.67), followed by lateral (mean mRS = 2.3), posterior (mean mRS = 2.7), and worst in massive BGHs (mean mRS = 4.83). In agreement with these results, the mean delta mRS (△ mRS = admission mean mRS - six-month mean mRS) showed the highest recovery potentials in anterior BGHs (△ mRS = 2.33), followed by lateral (△ mRS = 1.61), posterior (△ mRS = 0.94), and the least in massive BGHs (△ mRS = 0.09). Among borderline amount of BGH groups of lateral and posterior type, the mean mRS of posterior type was initially better than that of lateral type at the time of admission (3.64 vs. 3.92, respectively), but these results were reversed at six months after discharge (2.31 vs. 2.7, respectively) (Fig. 5).
In single logistic regression analysis, age, amount of hematoma, IVH, initial GCS, and massive type BGH were considered possible risk factors for poor outcome (mRS 4-6 at six months), while, after multiple logistic regression analysis, only age and initial GCS remained statistically significant risk factors (Table 3).
The anterior type was uncommon (9/103, 8.7%). This type mainly involves the caudate head and possibly extends to the anterior limb of the internal capsule and rostral part of the putamen (Fig. 1A). Hematoma was generally round in shape and the smallest mean volume was observed among subgroups of BGH, measured as 9.7 ± 8.6 mL. Hematoma expansion was not observed. Three patients (33.3%) had accompanying intraventricular hemorrhage (IVH), however, development of hydrocephalus did not occur in any case (Fig. 1B). None of the patients was assessed as below 8 points on the Glasgow coma scale (GCS) and eight patients (88.9%) were over GCS 13 on initial presentation. Two patients presented with poor motor weakness at admission, but all had recovered at discharge (Table 2). Only one patient underwent navigator assisted intracerebral hemorrhage (ICH) catheter insertion. There were no deaths.
The posterior type was most common (56/103, 54.4%) and mainly involved putamen, globus pallidus, and posterior limb of the internal capsule (Fig. 2). Hematoma tended to be irregular in shape and the mean volume was 26.3 ± 19.9 mL. Three patients had IVH but no progressive hydrocephalus. Hematoma expansion was observed in eight patients (14.3%). Thirty one patients (55.4%) underwent navigator assisted ICH catheter insertion and one patient underwent craniotomy and hematoma removal. Poor motor grade was demonstrated in 45 patients (80.4%) at admission, 41 patients (73.2%) at discharge, and 35 patients (62.5%) at six months after discharge (Table 2). One patient died due to hepatic encephalopathy within six months after discharge.
The second most common BGH was lateral type (26/103, 25.2%). The lateral BGH is located below the insular cortex and outside the putamen (Fig. 3A). Hematoma was generally ovoid in shape and the mean volume was 27.7 ± 18.3 mL (Fig. 3B). Hematoma expansion was observed in seven patients (26.9%), most commonly among the four subgroups. Four patients had IVH, but no further development of hydrocephalus was observed in any case. Most patients (17, 65.4%) showed a relatively good level of consciousness as over 13 GCS, and only one patient scored below 8 on GCS. Twenty patients (76.9%) had poor limb motor grade at the time of admission, which remained in 14 patients (53.8%) at follow-up check (Table 2). Eighteen patients (69.2%) underwent navigator assisted ICH catheter insertion and subsequent fibrinolytic treatment. There was no occurrence of mortality within six months after discharge.
Twelve patients (12/103, 11.7%) were massive type. This type occupied all three other regions (Fig. 4). Hematoma was generally irregular in shape and the largest mean volume was 115.8 ± 38.4 mL. Hematoma expansion was observed in two patients (41.7%). Accompanying IVH was observed in all cases and development of acute hydrocephalus was observed in two patients. The GCS of nine patients (75%) was below 8 points. All patients in this type presented with poor motor grade, which did not recovered until six months after discharge. Ten patients underwent surgical treatment; craniotomy and hematoma removal in four patients and navigator assisted ICH catheter insertion followed by fibrinolytic treatment in six patients (Table 2). Mortality rate was 33.3%. Two patients who did not receive surgical treatment expired before discharge.
BGHs differ variously in size, shape, and location, which may affect clinic-radiologic manifestations. We assumed that BGH could show some standardized pattern because the consistency of each nucleus of striatum, capsular fibers, and peri-ventricular matter is differently discrete, and, as a resultant, may act as a boundary of the hemorrhagic cavity. In the previous report by Chung et al.,4) BGHs were categorized according to six types based on vascular territory, including anterior, middle, posteromedial, posterolateral, lateral, and massive type. Each type presented with its own unique location, morphological characteristics, and clinical presentations and outcomes, similar to ours. However, in clinical application, it often seemed confusing and slightly cumbersome to differentiate one type of BGH from another type, particularly between middle, posteromedial, and posterolateral type. Therefore, we tried to categorize BGHs more simply into four subgroups mainly based on anatomical structures and could assure that this modified subgroup of BGH still reflect well enough their clinico-radiological characteristics.
The amount of hemorrhagic volume is the critical factor related to initial neurologic status and subsequent long term prognosis.5)8)15)16) Therefore, it was notable that the volume of hematoma showed a distinct trend according to its location. The largest mean amount of hematoma was observed in massive type, as intuitively expected, and anterior type showed the smallest volume. In addition, initial GCS score was the highest in anterior type, meaning that most patients with anterior BGHs are less neurologically stunned. In general, initial GCS score was slightly higher in posterior type than in lateral type, but posterior type showed a broad spectrum in GCS. Because the lateral and posterior type tended to show a moderate amount of hemorrhage (20~30mL), more sophisticated decision making regarding surgical intervention may be mandatory. According to our observation, the potentials for recovery from motor weakness were better in lateral type than in posterior type. In lateral type, motor weakness showed rapid recovery during hospitalization before discharge, however, in the posterior type, recovery of motor grade was gradual during long term rehabilitation after discharge. It appeared that the posterior type damaged basal ganglia and adjacent structures directly, but lateral BGH did not directly affect the neural tracts, but simply compressed them. For this reason, patients in lateral type BGH may require early surgical intervention for rescue of a salvageable neural tract. The well circumscribed, ovoid shape of lateral BGH is also best fit to stereotactic catheter aspiration and subsequent fibrinolytic treatment, whereas posterior BGHs are often shaped in an irregular staghorn. Steady and continuous rehabilitation could be emphasized more in patients with posterior BGH.
Previous reports have advocated that larger ICHs were significantly more irregular in shape, heterogeneous in density, and had greater growth, and density heterogeneity independently predicted ICH growth.1)9) In this study, a layered density of hematoma on initial CT scan showed correlation with hematoma expansion, as in a previous study. A layered density of hematoma on initial computed tomography was seen mainly in massive type. However, hematoma expansion among BGHs showing layered density was most frequently identified in the lateral type of BGH. This may be because emergent surgical intervention was more readily adopted in posterior and massive BGHs so that the time interval between initial and follow-up CT was too short to recognize identifiable hematoma expansion. Actually, hematoma expansion was observed in two patients who did not undergo surgical treatment. At least, we could assure that the layered density within the hematoma cavity is a strong predictor of hematoma growth.
IVH was seen mainly in massive type, followed by anterior type. Because the medial side of the caudate nucleus is composed of the lateral wall of the frontal horn of the ventricle, anterior BGHs can spread into the ventricle more frequently than posterior and lateral BGHs do. Many reports advocate that the combined IVH is a negative factor for good recovery,6)8)11) however, the existence of IVH appeared not to affect the final outcome if combined with anterior BGH. On the contrary, if IVH is combined with another type of BGH, it may implicate the explosive nature of hemorrhage, resulting in a relatively poor clinical course.
Regarding functional outcome on follow up check, the highest recovery potentials were observed in anterior BGHs, followed by lateral, posterior, and massive BGHs were most ominous. Among borderline amount of BGH groups of lateral and posterior type, it was interesting that the mean mRS of posterior type was initially better than the lateral type at the time of admission, but these results were reversed at six months after discharge. As previously described regarding recovery potentials in motor weakness, the initial insult by the larger volume of hematoma might reflect slightly poorer neurological status in lateral BGHs, however, simply stunned neurophysiological function in lateral BGHs could better retain their ability soon after alleviation of mechanical and chemical stresses than destructed neural pathway in posterior BGHs could do. The corticospinal tract, a major neural tract in the human brain for motor function, runs along the posterior limb of the internal capsule. Therefore, this neural pathway may be more preserved in anterior and lateral type BGH than posterior type BGH, which can affect functional outcome. As shown in Fig. 5, the mean mRS of lateral BGHs nearly catches up with that of the posterior BGHs at the time of discharge, and finally surpassed at six months after discharge. Among these two deep seated, medium volumes of ICH groups, the final prognosis might differ as to the primary locations of hematoma.
Except for patients in massive type, no patient died due to BGH itself. The cause of death in one patient in posterior type was hepatic encephalopathy. Two patients who did not undergo surgical treatment in massive type expired before discharge. One patient with massive type who underwent direct surgical evacuation of hematoma recovered and scored mRS 2 at six months after discharge. Although there is significant criticism regarding performance of surgical intervention in deep seated ICHs,2)3)12)13)17) we experienced and believed that a certain patient may still possess recovery potential. Over 75% of patients with massive BGHs were unconscious, uncommunicable in devastating status, thus, selection of patients who might benefit from surgical intervention seemed difficult. Therefore, an aggressive strategy to include all possible surgical candidates can be the final opportunity for recovery for life threatening ICH victims, once providing that the surgical intervention can meet the condition of 'at least do no harm'.
This is a retrospective study, harboring possible bias in patient selection, assessment, and follow-up plan. Furthermore, we did not assess additional clinical manifestations, such as language impairment, cognitive function, and other non-motor deficits. In addition, because variables between hematoma volume, initial GCS, IVH, and type of BGH may be co-related, and the number of data in each group seems relatively small, the statistical calculation failed to show significance of regional location of BGH in assessing risk factors for poor outcome. All of these are possible limitations of this report, and subject to future investigation.
References
1. Barras CD, Tress BM, Christensen S, MacGregor L, Collins M, Desmond PM, et al. Density and shape as CT predictors of intracerebral hemorrhage growth. Stroke. 2009; 4. 40(4):1325–1331. PMID: 19286590.

2. Batjer HH, Reisch JS, Allen BC, Plaizier LJ, Su CJ. Failure of surgery to improve outcome in hypertensive putaminal hemorrhage. A prospective randomized trial. Arch Neurol. 1990; 10. 47(10):1103–1106. PMID: 2222242.
3. Benes L, Nimsky C. Management of supratentorial intracerebral hemorrhage-still a controversy? World Neurosurg. 2012; 1. 77(1):55–56. PMID: 22154149.

4. Chung CS, Caplan LR, Yamamoto Y, Chang HM, Lee SJ, Song HJ, et al. Striatocapsular haemorrhage. Brain. 2000; 9. 123(Pt 9):1850–1862. PMID: 10960049.

5. Dennis MS. Outcome after brain haemorrhage. Cerebrovasc Dis. 2003; 16(Suppl 1):9–13. PMID: 12698013.

6. Flaherty ML, Haverbusch M, Sekar P, Kissela B, Kleindorfer D, Moomaw CJ, et al. Long-term mortality after intracerebral hemorrhage. Neurology. 2006; 4. 66(8):1182–1186. PMID: 16636234.

7. Ghika JA, Bogousslavsky J, Regli F. Deep perforators from the carotid system. Template of the vascular territories. Arch Neurol. 1990; 10. 47(10):1097–1100. PMID: 2222241.
8. Hansen BM, Nilsson OG, Anderson H, Norrving B, Saveland H, Lindgren A. Long term (13 years) prognosis after primary intracerebral haemorrhage: a prospective population based study of long term mortality, prognostic factors and causes of death. J Neurol Neurosurg Psychiatry. 2013; 10. 84(10):1150–1155. PMID: 23715913.

9. Kazui S, Naritomi H, Yamamoto H, Sawada T, Yamaguchi T. Enlargement of spontaneous intracerebral hemorrhage. Incidence and time course. Stroke. 1996; 10. 27(10):1783–1787. PMID: 8841330.
10. Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996; 8. 27(8):1304–1305. PMID: 8711791.

11. Kumral E, Evyapan D, Balkir K. Acute caudate vascular lesions. Stroke. 1999; 1. 30(1):100–108. PMID: 9880396.

12. Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the international surgical trial in intracerebral haemorrhage (STICH): a randomised trial. Lancet. 2005; Jan-Feb. 365(9457):387–397. PMID: 15680453.

13. Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet. 2013; 8. 03. 382(9890):397–408. PMID: 23726393.

14. Middleton FA, Strick PL. Basal ganglia output and cognition: evidence from anatomical, behavioral, and clinical studies. Brain Cogn. 2000; 3. 42(2):183–200. PMID: 10744919.

15. Nagaratnam N, Saravanja D, Chiu K, Jamieson G. Putaminal hemorrhage and outcome. Neurorehabil Neural Repair. 2001; 15(1):51–56. PMID: 11527279.

16. Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001; 5. 344(19):1450–1460. PMID: 11346811.

17. Talacchi A, Ricci UM, Caramia G, Massimo G. Basal ganglia haemorrhages: efficacy and limits of different surgical strategies. Br J Neurosurg. 2011; 4. 25(2):235–242. PMID: 21158512.

18. van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010; 2. 9(2):167–176. PMID: 20056489.

Fig. 1
Anterior basal ganglia hemorrhage (BGH) in computed tomography (CT) images. (A) Axial non-contrast CT scan shows a small, round shaped hemorrhage in the right caudate nucleus without circumferential extension. (B) A small amount of hemorrhage in the left caudate head extends into the ipsilateral lateral ventricle.
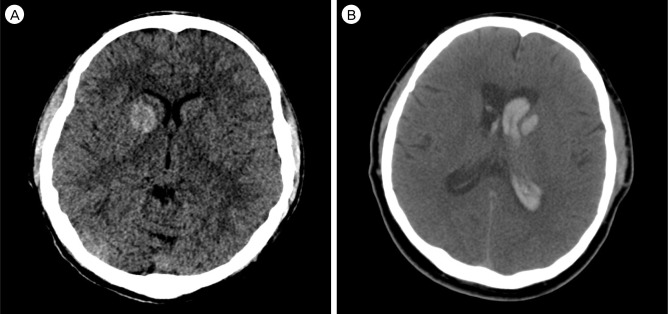
Fig. 2
Posterior basal ganglia hemorrhage (BGH) in computed tomography (CT) images. (A) Axial non-contrast CT scan shows a small amount of hemorrhage well confined in the left putamen. (B) An irregular shaped putaminal hemorrhage extends to the lateral border of the thalamus and posterior limb of the internal capsule. (C) Posterior BGH involving putamen, globus pallidus, and posterior limb of the internal capsule.
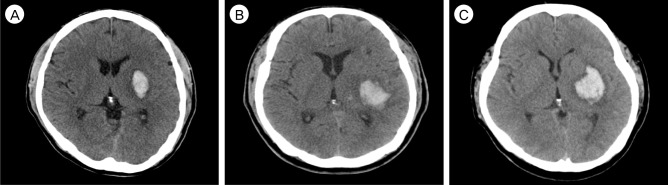
Fig. 3
Lateral basal ganglia hemorrhage (BGH) in computed tomography (CT) images. (A) Axial non-contrast CT scan shows medium to large amount of hemorrhage beneath the insular cortex. The striato-capsular structures are not involved, but compressed and shifted. (B) The lateral BGH representatively in elongated, ovoid shape.
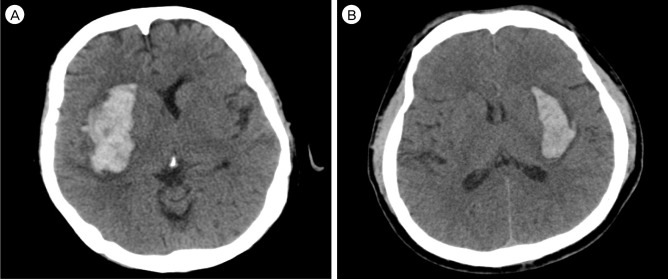
Fig. 4
Massive basal ganglia hemorrhage (BGH) in computed tomography (CT) images. Large amount of hemorrhage occupied all striato-capsular regions and extended to the lateral ventricle. Anatomic structures are distorted and shifted.
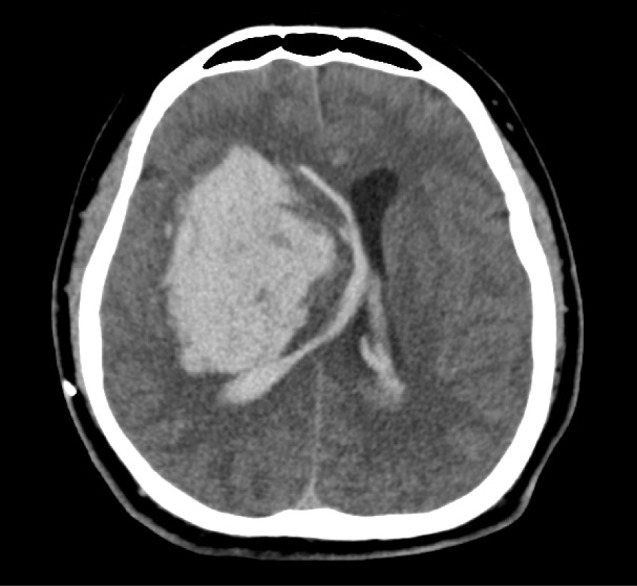
Fig. 5
The mean modified Rankin scale scores (mRS) of each group of basal ganglia hemorrhage (BGH) at the time of admission, discharge, and six months after discharge are shown. The mean mRS at the time of admission was the best in anterior BGH (= 3.0), followed by posterior (= 3.64), lateral (= 3.92), and the worst in massive BGH (= 4.92). At discharge, the mean mRS was 1.56 in anterior BGH, 3.27 in posterior, 3.38 in lateral, and 5.17 in massive BGH. At six-month follow up, the mean mRS was the best in anterior BGH (= 0.67), followed by lateral (= 2.31), posterior (= 2.7), and the worst in massive BGH (= 4.83). The mean delta mRS (△ mRS = admission mean mRS - six-month mean mRS) revealed the highest recovery potentials in anterior BGHs (= 2.33), followed by lateral (= 1.61), posterior (= 0.94), and the least in massive BGHs (= 0.09).




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download