Abstract
Objective
In the so-called primary intracerebral hemorrhage (ICH), lobar and deep ICH were mainly due to cerebral amyloid angiopathy and deep perforating arterial disease. Our aim was to identify specifics of warfarin associated ICH (WAICH) and to focus on differences in susceptibility to warfarin according to the underlying vasculopathies, expressed by ICH location.
Materials and Methods
We identified all subjects aged ≥ 18 years who were admitted with primary ICH between January 1, 2007 and September 30, 2012. We retrospectively collected demographic characteristics, the presence of vascular risk factors and pre-ICH medication by chart reviews. We categorized ICH into four types according to location: lobar, deep, posterior fossa, and undetermined. We investigated characteristics (including hematoma volume and expansion) of ICH according to the location of ICH.
Results
WAICH accounted for 35 patients (5.6%) of 622 ICH cases. In WAICH, 13 patients (37.1%) had lobar ICH and 22 patients (60.0%) had non-lobar ICH. Compared to other locations of ICH, lobar ICH showed an excess risk of WAICH (OR 2.53, 95% CI 1.03-6.21, p = 0.042). The predictors of lobar location of ICH were warfarin (OR 2.29, 95% CI 1.05-5.04, p = 0.038) and diabetes mellitus (DM) (OR 0.54, 95% CI 0.29-0.98, p = 0.044). The lobar location of ICH showed significant association with larger hematoma volume (p = 0.001) and high ratio of hematoma expansion (p = 0.037) compared with other locations of ICH.
The high prevalence of atrial fibrillation in the aging population has led to an increasing incidence of warfarin associated intracerebral hemorrhage (WAICH).10)21) Although WAICH is predictive of larger hematoma volume and high rates of hematoma expansion and worse clinical outcome than primary ICH, the mechanisms of these results were not completely understood.5)11)
Many diseases may lead to ICH; two prominent causes of primary ICH are cerebral amyloid angiopathy in lobar ICH and deep perforating artery vasculopathy in deep ICH.2)20) This was indicated with autopsy in prognosis of an ICH (PITCH) cohort.7) The impact of warfarin on these types of ICH was distinct, which suggests different sensibilities of these two vasculopathies on warfarin. To date, only Nelly's article reported on the impact of warfarin according to the underlying vasculopathies.7) This article reported an association of vitamin K antagonists use with larger hematoma volume only in non-lobar ICH.7)
We aimed at identification of specifies of WAICH and focused on differences in susceptibility to warfarin according to the underlying vasculopathies, expressed by the ICH location.
We identified all subjects aged ≥ 18 years who were admitted with primary ICH to Busan-Ulsan Regional Cardiocerebrovascular Center between January 1, 2007 and September 30, 2012. Exclusion criteria were traumatic ICH, hemorrhagic transformation of cerebral infarction, ICH secondary to vascular malformation, tumor, aneurysm, and vasculitis of the central nervous system. In addition, we excluded patients with primary intraventricular hemorrhage (IVH), liver cirrhosis, and other coagulopathies.
We retrospectively collected demographic characteristics, medical history of vascular risk factors such as hypertension (HTN), diabetes mellitus (DM), smoking, hyperlipidemia, previous stroke, atrial fibrillation (AF), ischemic heart disease, congestive heart failure (CHF), heart valve disease, pulmonary thromboembolism (PTE), liver disease, chronic kidney disease (CKD), and pre-ICH medication by chart review.
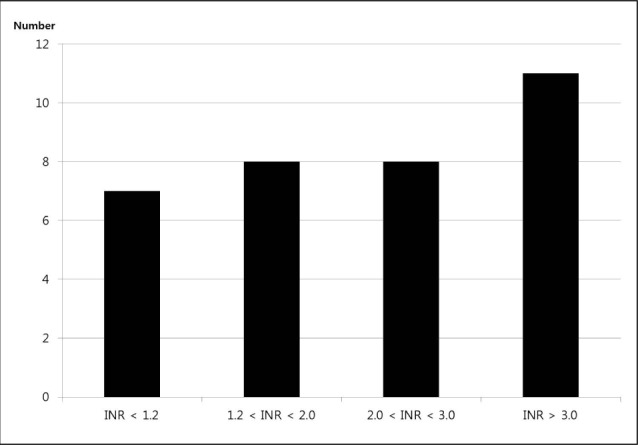
We assessed the severity of neurological deficit by Glasgow coma scale (GCS) and measured the international normalized ratio (INR) value at admission. For WAICH patients, the INR values obtained before reversals were used for this report. WAICH patients were stratified by level of anticoagulation (INR < 1.2, 1.2-2.0, 2.0-3.0, > 3.0).
We checked computed tomography (CT) scans at admission and after 4 hours in all ICH patients, with continuous slices, no gap, and 5 mm slice thickness. We distributed the location of ICH: (1) lobar (frontal, temporal, parietal, and occipital) when the origin of hemorrhage appeared to be in the cerebral hemisphere superficial to the deep gray matter structure; (2) deep when originating from basal ganglia and thalamus (3) in the posterior fossa when originating from the brain stem or cerebellum and (4) undetermined in case of ICH when the origin could not be identified and including huge ICH cases that overlapped two categories. We calculated the volume of ICH according to the ABC/2 method.19) The expansion of hematoma was defined as increasing by 33% and 6 cc in hematoma volume over 4 hours.
To ascertain factors associated with warfarin, we used χ2-test and independent samples t-test for comparison of average values in all ICH patients. We explored the influence of warfarin on volume and hematoma expansion, reflecting the influence of the ICH location (Lobar vs Deep). Therefore, intending lobar and deep ICH, we performed multiple linear regression analysis including the location of ICH, taking warfarin, and others. After dividing these ICH patients according to the location of ICH, we repeated multiple linear regression analysis. In lobar and deep ICH, multiple logistic regression analysis was performed to estimate the independent contributions of variables. Statistical analysis was performed using SPSS version 12.0 (SPSS Inc., Chicago, IL, United States). All p values less than 0.05 were considered statistically significant.
We included 622 patients (Median age, 52; interquartile range [IQR], 33-76), 348 were men (55.9%). Thirty five patients (5.6%) were taking warfarin at admission. Fourteen patients (40%) took warfarin for heart valve diseases, seven (20%) for AF, seven (20%) for ischemic stroke, four (11%) for PTE, and three (9%) for venous thromboembolism. The characteristics of patients taking warfarin versus those not taking warfarin are shown in Table 1. In univariate analysis, factors associated with WAICH patients were older age, comorbidities such as AF, history of previous ischemic stroke and pre-ICH medication (antiplatelet and statin). Patients taking warfarin had a more severe neurologic defect at admission: median GCS 12 (9-14) in the not taking warfarin group versus eight in WAICH (6-11), however, there was no statistically significant difference (p = 0.134).
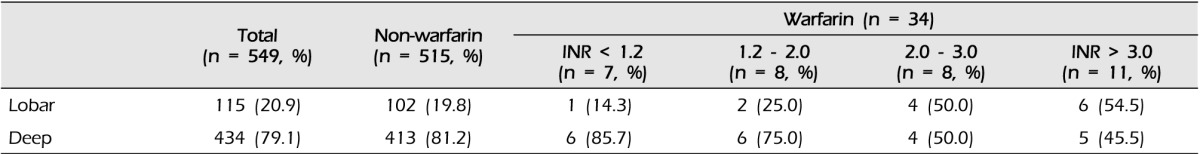
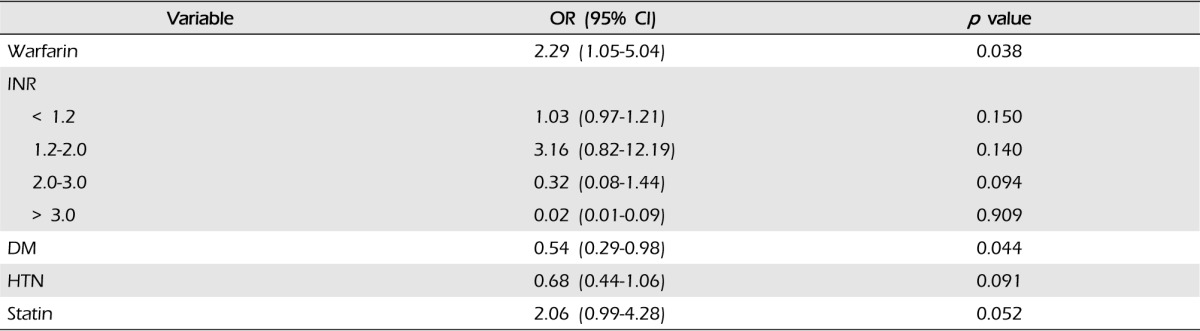
At admission, the median INR value was 2.1 (IQR 1.2-3.3) in all WAICH patients, 2.9 in lobar (IQR, 2.1-3.6), 1.7 in deep (IQR, 1.0-2.9), and 0.9 in posterior fossa (p = 0.202). The numbers of WAICH patients categorized according to the INR value and are shown in Fig. 1. Among patients reported to be taking warfarin, only eight (23.8%) patients had therapeutic INR (2.0-3.0). The categories of INR value classified according to the location of ICH are shown in Table 2. The number of lobar ICH patients in WAICH whose INR value under 2.0 was three (20%), while that of the lobar patients whose INR value over 2.0 was 10 (52.6%) (p = 0.051).
Taking warfarin showed significant association with a larger volume of ICH compared with the other group (p < 0.001) (Fig. 2). In multiple linear regression on all documented patients, the only predictor of larger hematoma was the location of ICH (p = 0.001). After sorting our cohorts according to the location of ICH, we performed multiple linear regression analysis (Table 3) ; both in lobar ICH and in deep ICH, patients taking warfarin showed larger hematoma volume (p < 0.001, p < 0.001). To elucidate a dose-response relationship between INR value and volume of ICH, we performed an analysis with INR categories. We found that patients with higher INR value expressed larger ICH in all ICH patients (p = 0.011); in lobar ICH, INR values showed an association with the volume (p = 0.031), but in deep ICH, the volume was not influenced by INR values (p = 0.239) (Fig. 3).
In our cohorts, more than 33% of hematoma volume was increased in 144 cases and hematoma volume of 6 cc or more was increased in 92 cases. In our cohorts, 44 cases showed a hematoma increase of both percentage and volume.
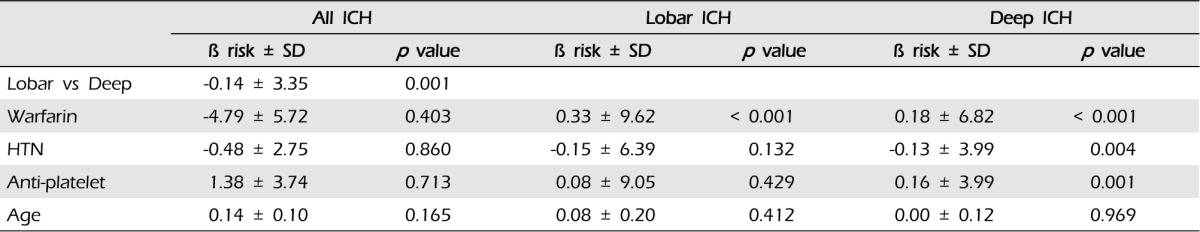
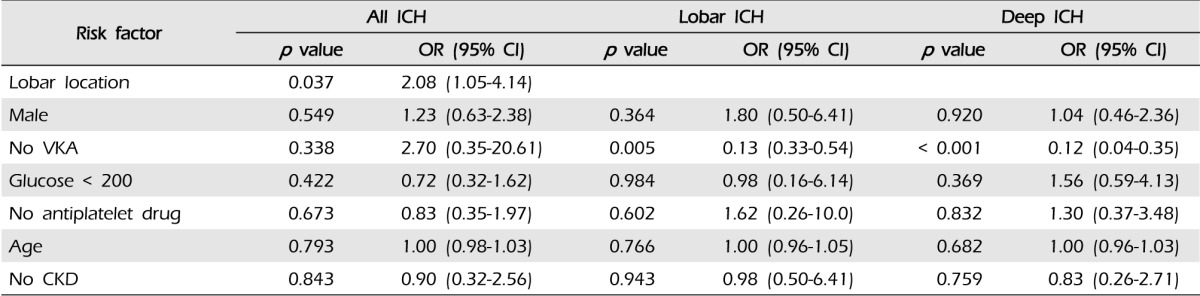
Taking warfarin showed significant association with the expansion of ICH (p < 0.001). In our cohorts, the only predictor of expansion was the lobar location of ICH (p = 0.037, OR 2.08, 95% CI:1.05-4.14). After dividing patients according to the location of ICH, we performed multiple linear regression analysis (Table 4): (1) in lobar ICH, patients under warfarin showed more hematoma expansion (p = 0.001); and (2) in non-lobar ICH patients, taking warfarin also influenced the expansion of ICH (p < 0.001) (Fig. 4).
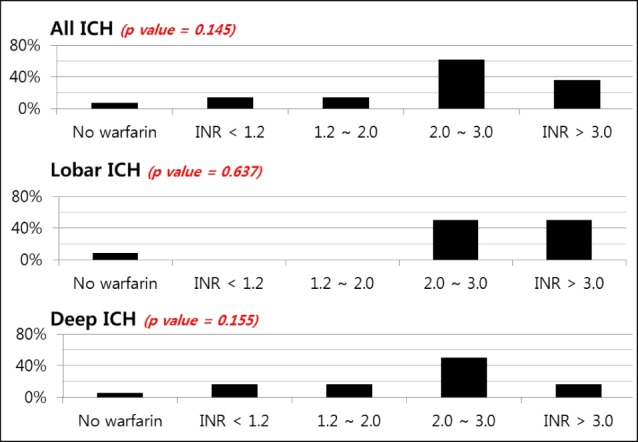
To elucidate a dose-response relationship between INR value and the ratio of expansion in ICH, we performed an analysis with INR categories. We found that higher INR value was not associated with the expansion of hematoma in all ICH patients (p = 0.145). After dividing the location of ICH, expansion of hematoma was not affected by INR value (Fig. 5).
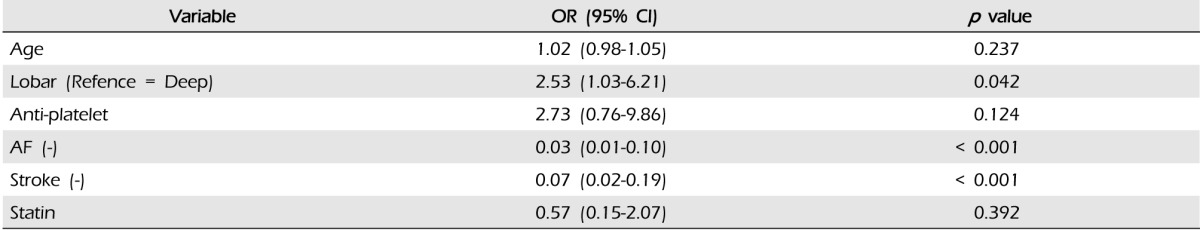
Results of multivariate logistic regression analysis of the risk factors in WAICH are shown in Table 5. History of AF and ischemic stroke showed strong association with WAICH. Compared with other ICH locations, only lobar location showed an excess risk of WAICH (OR 2.53, 95% CI 1.03-6.21). The history of antiplatelet medication showed the highest odds ratio in our cohort, but did not reach statistical significance. (p = 0.124, OR 2.73, 95% CI 0.76-9.86) The results of multivariate analysis of lobar ICH are shown in Table 6. A history of DM and taking warfarin were significant risk factors of lobar ICH. Although taking warfarin was the most important risk factor of lobar ICH, intensity of anticoagulation did not affect the lobar location of ICH.
Approximately 1% of the population is currently taking warfarin and this ratio has shown an increase in recent studies.14) Previous studies reported that 5~12% of ICH occurred by oral anticoagulation.6)14)18) Patients with WAICH had worse outcome.7)11) In our study, we have found the relationship between WAICH and underlying vasculopathies such as amyloid angiopathy and deep perforating arterial disease, which may contribute to the location of ICHs.
In previous reports, advanced age, HTN, history of cerebrovascular disease, and intensity of anticoagulation (mainly if INR > 4.0) were predisposing factors of WAICHs.1)14) Consistent with previous reports, we found that WAICH patients were older and had more vascular risk factors compared with the other group. Although value of INR more than 4.0 was a significant risk factor of WAICH, most WAICH occurred at INR < 3.0.9)17) In our study, 60% of our patients were within or below the therapeutic INR range and median INR value was 2.2 (IQR 1.2-3.3). In patients taking warfarin, as well as in the normal range INR value, physicians should pay attention.
In two previous reports, larger baseline volume of hematoma in WAICH was the major difference as compared to primary ICH.4)23) We also found that patients using warfarin had larger hematoma volume. The relationship between anticoagulation intensity measured on admission and hematoma volume was controversial. Flaherty's study reported that only INR > 3.0 showed an association with larger hematoma volume, however, another study published by Berwaert reported that larger hematomas did not show correlation with higher INR value.3)11) In our study, significant association was observed between the anticoagulation intensity measured on admission and the hematoma volume, like other reports.15)
In patients taking warfarin, we found that the hematoma volume differed depending on the location of ICH. In our study, the lobar location was the most important factor affecting the volume of ICH and the intensity of anticoagulation was relative to the volume of ICH only in lobar ICH. However, Nelly's cohort study, the only paper reporting on the effects of vitamin K antagonist (VKA) on the volume according to location, showed opposites results; deep ICH, caused by vasculopathy of deep perforating arteries, which seems to lead to larger ICH in VKAs use.7) On the other hand, Rosand's study reported that cerebral amyloid angiopathy was very sensitive to antithrombotics; he identified lobar hemorrhage as the predictor of larger baseline volume.25) Because our report and other studies were limited by inclusion of a small number patients and retrospective reports, prospective extensive research was required in order to confirm a significant association between lobar ICH and larger hematoma volume.
In previous studies, patients taking warfarin at the time of their ICH were associated with increased risk for hematoma expansion.5) History of HTN, hyperglycemia, the lobar location of ICH, and chronic kidney disease were found to show association with greater ICH expansion in some studies.12)27) The INTERACT1 study reaffirmed the importance of hematoma expansion as a dominant contributor to death and dependency in ICH.6) Every 1 ml of hematoma expansion was estimated to be associated with a 5% increase in odds of death and dependency.6) In our study, the intensity of warfarin was not associated with the expansion of ICH. We also found that the location of ICH was the most important factor in hematoma expansion. After dividing patients according to the location of ICH, warfarin was a strong risk factor for hematoma expansion. These results supported that the lobar ICH in patients taking warfarin was expected to have a greater possibility of hematoma expansion.
Results of studies on the location of WAICH have been controversial. Some reports showed a higher proportion of lobar and thalamic hemorrhage in patients taking warfarin.16)24) On the other hand, another study found that taking warfarin was highly associated with the location of cerebellum, particularly in patients with an INR > 2.5.26) In our study, the lobar location of ICH was a significant factor of WAICH and the most important risk factor of lobar hemorrhage was also taking warfarin.
Our study has many limitations. This study had a retrospective design, including the small numbers of WAICH. Time from neurologic symptom onset to initial CT scan may affect the volumes of ICH, however, we did not consider this in our cohort study. In addition, our report used the ABC/2 method for calculation of hematoma volume. This method has been reported to significantly overestimate volume, especially when the shape of hematoma was irregular, which was frequent in WAICH. Another limitation in our study was that the median INR values in each location of WAICH were different. Nevertheless, of the limited size of sample and the different baseline INR, our results showed a strong association between warfarin and lobar ICH, resulting in the larger incidence, initial volume, and expansion of hematoma. Conduct of a prospective larger scale study is necessary in order to understand the exact characteristics of WAICH according to the underlying vasculopathy.
In our study, taking warfarin showed a signification association with the occurrence, volume of hematoma, and expansion of lobar ICH. In addition, the intensity of anticoagulation is associated with larger hematoma volume in lobar ICH. In patients suspected as having high risk factor for lobar ICH, such as amyloid angiopathy, attention should be paid to those taking warfarin.
References
1. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994; 7. 154(13):1449–1457. PMID: 8018000.
2. Al-Shahi Salman R, Labovitz DL, Stapf C. Spontaneous intracerebral haemorrhage. BMJ. 2009; 7. 24. 339:b2586. PMID: 19633038.

3. Berwaerts J, Dijkhuizen RS, Robb OJ, Webster J. Prediction of functional outcome and in-hospital mortality after admission with oral anticoagulant-related intracerebral hemorrhage. Stroke. 2000; 11. 31(11):2558–2562. PMID: 11062275.

4. Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993; 7. 24(7):987–993. PMID: 8322400.

5. Cucchiara B, Messe S, Sansing L, Kasner S, Lyden P. CHANT Investigators. Hematoma growth in oral anticoagulant related intracerebral hemorrhage. Stroke. 2008; 11. 39(11):2993–2996. PMID: 18703803.

6. Delcourt C, Huang Y, Arima H, Chalmers J, Davis SM, Heeley EL, et al. INTERACT1 Investigators. Hematoma growth and outcomes in intracerebral hemorrhage: the INTERACT1 study. Neurology. 2012; 7. 79(4):314–319. PMID: 22744655.

7. Dequatre-Ponchelle N, Hénon H, Pasquini M, Rutgers MP, Bordet R, Leys D, et al. Vitamin K antagonists-associated cerebral hemorrhages: what are their characteristics? Stroke. 2013; 2. 44(2):350–355. PMID: 23287784.
8. Diringer MN, Skolnick BE, Mayer SA, Steiner T, Davis SM, Brun NC, et al. Thromboembolic events with recombinant activated factor VII in spontaneous intracerebral hemorrhage: results from the Factor Seven for Acute Hemorrhagic Stroke (FAST) trial. Stroke. 2010; 1. 41(1):48–53. PMID: 19959538.
9. Fang MC, Chang Y, Hylek EM, Rosand J, Greenberg SM, Go AS, et al. Advanced age, anticoagulation intensity, and risk for intracranial hemorrhage among patients taking warfarin for atrial fibrillation. Ann Intern Med. 2004; 11. 141(10):745–752. PMID: 15545674.

10. Flaherty ML, Kissela B, Woo D, Kleindorfer D, Alwell K, Sekar P, et al. The increasing incidence of anticoagulant-associated intracerebral hemorrhage. Neurology. 2007; 1. 68(2):116–121. PMID: 17210891.

11. Flaherty ML, Tao H, Haverbusch M, Sekar P, Kleindorfer D, Kissela B, et al. Warfarin use leads to larger intracerebral hematomas. Neurology. 2008; 9. 71(14):1084–1089. PMID: 18824672.

12. Flibotte JJ, Hagan N, O'Donnell J, Greenberg SM, Rosand J. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology. 2004; 9. 63(6):1059–1064. PMID: 15452298.

13. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke : results from the National registry of atrial fibrillation. JAMA. 2001; 6. 285(22):2864–2870. PMID: 11401607.
14. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007; 6. 146(12):857–867. PMID: 17577005.

15. Huttner HB, Schellinger PD, Hartmann M, Kohrmann M, Juettler E, Wikner J, et al. Hematoma growth and outcome in treated neurocritical care patients with intracerebral hemorrhage related to oral anticoagulant therapy: comparison of acute treatment strategies using vitamin K, fresh frozen plasma, and prothrombin complex concentrates. Stroke. 2006; 6. 37(6):1465–1470. PMID: 16675739.
16. Itabashi R, Yasaka M, Kuwashiro T, Nakagaki H, Miyashita F, Naritomi H, et al. Location of acute brain hemorrhage in patients undergoing antithrombotic therapy. J Neurol Sci. 2009; 5. 280(1-2):87–89. PMID: 19254798.

17. Jeffree RL, Gordon DH, Sivasubramaniam R, Chapman A. Warfarin related intracranial haemorrhage: a case-controlled study of anticoagulation monitoring prior to spontaneous subdural or intracerebral haemorrhage. J Clin Neurosci. 2009; 6. 16(7):882–885. PMID: 19342242.

18. Jorgensen HS, Nakayama H, Reith J, Raaschou HO, Olsen TS. Stroke recurrence: predictors, severity, and prognosis. The Copenhagen stroke study. Neurology. 1997; 4. 48(4):891–895. PMID: 9109873.

19. Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996; 8. 27(8):1304–1305. PMID: 8711791.

20. Labovitz DL, Boden-Albala B, Hauser WA, Sacco RL. Lacunar infarction or deep intracerebral hemorrhage: who gets which? The northern Manhattan study. Neurology. 2007; 2. 68(8):606–608. PMID: 17310033.
21. Lovelock CE, Molyneux AJ, Rothwell PM. Oxford vascular study. Change in incidence and etiology of intracerebral haemorrhage in Oxfordshire, UK, between 1981 and 2006: a population-based study. Lancet Neurol. 2007; 6. 6(6):487–493. PMID: 17509483.
22. Mittal MK, Rabinstein AA. Anticoagulation-related intracranial hemorrhages. Curr Atheroscler Rep. 2012; 8. 14(4):351–359. PMID: 22638877.

23. Nilsson OG, Lindgren A, Brandt L, Saveland H. Prediction of death in patients with primary intracerebral hemorrhage: a prospective study of a defined population. J Neurosurg. 2002; 9. 97(3):531–536. PMID: 12296635.

24. Radberg JA, Olsson JE, Radberg CT. Prognostic parameters in spontaneous intracerebral hematomas with special reference to anticoagulant treatment. Stroke. 1991; 5. 22(5):571–576. PMID: 2028484.

25. Rosand J, Eckman MH, Knudsen KA, Singer DE, Greenberg SM. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med. 2004; 4. 164(8):880–884. PMID: 15111374.

26. Toyoda K, Okada Y, Ibayashi S, Inoue T, Yasumori K, Fukui D, et al. Antithrombotic therapy and predilection for cerebellar hemorrhage. Cerebrovasc Dis. 2007; 23(2-3):109–116. PMID: 17124390.

27. Zubkov AY, Mandrekar JN, Claassen DO, Manno EM, Wijdicks EF, Rabinstein AA. Predictors of outcome in warfarin related intracerebral hemorrhage. Arch Neurol. 2008; 10. 65(10):1320–1325. PMID: 18852345.

Fig. 2
Median intracerebral hemorrhage volume between patients with and without warfarin. ICH = intracerebral hemorrhage
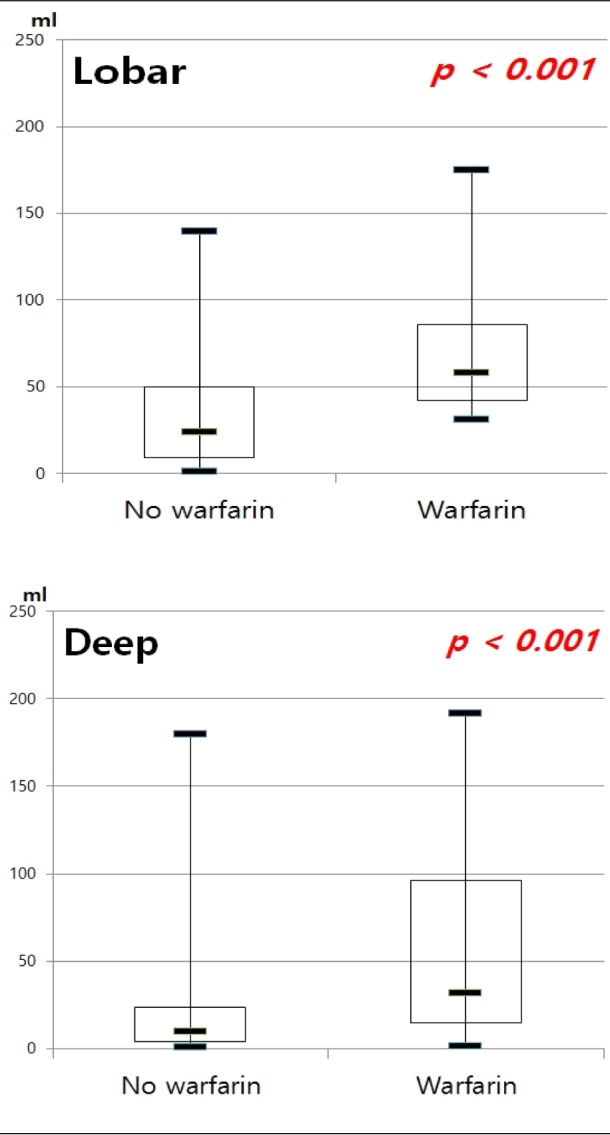
Fig. 3
Volume of ICH by location and level of anticoagulation. ICH = intracerebral hemorrhage; INR = international normalized ratio; OR = odds ratio
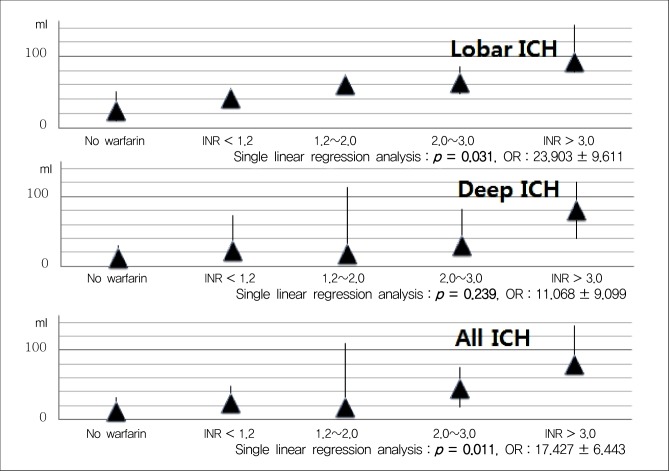
Fig. 4
Ratio of hematoma expansion between patients with and without warfarin. ICH = intracerebral hemorrhage
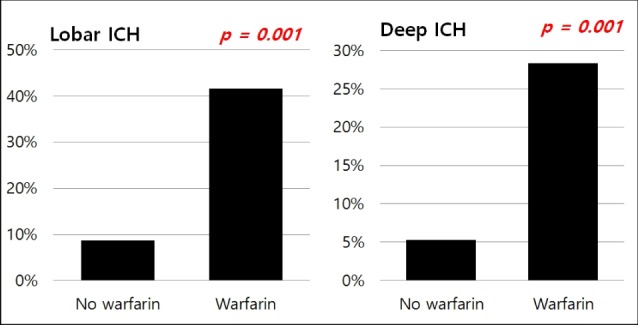
Fig. 5
Expansion of ICH by location and level of anticoagulation. ICH = intracerebral hemorrhage; INR = international normalized ratio




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download