Abstract
The pipeline™ embolization device (PED) is a braided, tubular, bimetallic endoluminal implant used for occlusion of intracranial aneurysms through flow disruption along the aneurysm neck. The authors report on two cases of giant internal carotid artery aneurysm treated with the PED. In the first case, an aneurysm measuring 26.4 mm was observed at the C3-C4 portion of the left internal carotid artery in a 64-year-old woman who underwent magnetic resonance imaging (MRI) for dizziness and diplopia. In the second case, MRI showed an aneurysm measuring 25 mm at the C4-C5 portion of the right internal carotid artery in a 39-year-old woman with right ptosis and diplopia. Each giant aneurysm was treated with deployment of a PED (3.75 mm diameter/20 mm length and 4.5 mm diameter/25 mm length, respectively). Nine months later, both cases showed complete radiological occlusion of the giant intracranial aneurysm and sac shrinkage. We suggest that use of the PED can be a therapeutic option for giant intracranial aneurysms.
The natural history of giant intracranial aneurysms includes high morbidity and mortality from rupture.27) Symptomatic lesions can be present in 50% of rupture cases, and the mortality rate is 60% in two years.6) These aneurysms are often found in the cavernous and paraclinoid segments of the internal carotid artery (ICA).2) In endovascular treatment of giant aneurysms using coils, performance of complete packing is difficult, and the rates of recurrence are high.3)7)18)25) Although advanced coil techniques using a double catheter, assisted balloon or stent, and complex-shaped coils have improved the radiological complete occlusion, 7~26% recanalization of the aneurysm has been reported in several cases.4)8)21)
The pipeline™ embolization device (PED; eV3/Covidien, Plymouth, MN, USA) has been introduced as a new modality for diversion of flow from the parent artery into the sac. It is different from the traditional concept of packing in the sac for a barrier with coils. This device induces a compromise of the hemodynamic flow between the parent artery and aneurysm, producing spontaneous thrombosis in the sac and neointimal growth in the neck.11)12)14)23) We report on our experience with two cases in which the PED was used for treatment of giant internal carotid artery (ICA) aneurysms for the first time in Korea.
A 64-year-old woman presented with left ptosis and lateral gaze restriction. Magnetic resonance imaging (MRI) showed a partially thrombosed giant aneurysm at the cavernous portion. Cerebral angiography showed that the maximal size and neck size of the aneurysm were 26.4 mm and 8.3 mm, respectively, at the C3-C4 segment of the ICA. We performed the balloon-occlusion test (BOT) with single photon emission computed tomography (SPECT), which showed that the collateral flow was not sufficient. The patient was referred for Pipeline™ stenting. Under general anesthesia, a 7-French Flexor® Shuttle® guiding sheath (Cook Medical, Bloomington, IN, USA) was placed in the right ICA, and a 6-French Envoy® guiding catheter (Cordis Corporation, Bridgewater, NJ, USA) was placed coaxially through the shuttle for increased support. The Marksman® 0.027 microcatheter (ev3/Covidien, Plymouth, MN, USA) was navigated to the left middle cerebral artery using a Traxcess® 0.014 microguidewire (Microvention, Tustin, CA, USA). A PED (diameter, 3.75 mm; length, 20 mm; ev3/Covidien, Plymouth, MN, USA) was introduced and deployed, spanning the aneurysm neck and achieving maximal apposition to the ICA wall. Final angiography showed a decreased vortex flow in the aneurysm sac without thromboembolic complications. Three-month follow-up, computerized tomography angiography (CTA) showed that the PED has a good apposition to the vessel wall. Six-month follow-up angiography showed that the vortex flow into the sac had nearly disappeared and the vessel wall had been reconstructed using the PED. Nine months later, CTA showed progressive shrinkage of the aneurysm sac, and the symptoms of ptosis and diplopia showed slight improvement (Fig. 1).
A 39-year-old woman presented with dizziness and intermittent diplopia. MRI showed a large (20.2 mm) aneurysm at the cavernous portion. During follow-up, she reported severe headache and continuous diplopia. CTA and cerebral angiography showed that the aneurysm had increased in size since one year earlier. The maximal size and neck size were 26.7 mm and 6.4 mm, respectively, at the C4 segment of the ICA. During BOT, the patient reported visual disturbance during the hypotensive challenge test. She was also referred for Pipeline™ stenting. The PED (diameter, 4.5 mm; length, 25 mm; ev3/Covidien, Plymouth, MN, USA) was introduced and deployed, spanning the aneurysm neck. Post-angiography showed a well-implanted PED with contrast stasis in the aneurysm sac. No treatment-related complications were noted. After three months, CTA showed contrast medium filling a small portion of the sac. Six months later, cerebral angiography showed that the parent artery sustained a good flow patency and vessel reconstruction; the residual small filling portion disappeared within nine months, however, the overall size was not changed with thrombosis. Fortunately, sixth nerve palsy showed slight improvement (Fig. 2).
Patients were pretreated with aspirin (100 mg/day) and clopidogrel (75 mg/day) for one week before PED deployment. During the procedure, intravenous heparin was administered at 50-100 U/kg for achievement of an activated clotting time of 250-300 seconds; this therapy was continued for 12 hours. After stenting, intravenous dexamethasone was administered at 20 mg/day for four days; subsequently, oral prednisolone was administered. Within 10 days, the oral medication was tapered as follows: 20 mg for six days, 15 mg for two days, 10 mg for one day, and 5 mg for one day. Dual antiplatelet medication was maintained for six months, and mono-medication (aspirin or clopidogrel) was prescribed indefinitely.
Interventionists encounter many difficulties in treatment of giant intracranial aneurysms. In the early endovascular treatment using only the coiling modality, retreatment was frequently required because complete occlusion of the aneurysm could not be achieved.7)18)25) Although combined use of a stent and balloon has improved the initial technique, the results are often not sustained because these aneurysms tend to require coil compaction and recanalization.3)4)8)21) In addition, in most cases, the orifice of the giant aneurysm is not a focal defect of the parent vessel wall but a segment of the parent vessel wall. In such cases, the conventional embolization technique, which involves filling the sac and covering the neck in order to disrupt flow into and out of the aneurysm, has a low efficacy for treatment of these circumferentially involved vessels.
The PED (ev3/Covidien, Plymouth, MN, USA), a braided, tubular, bimetallic endoluminal implant composed of 48 individual microfilaments prepared using 25% platinum and 75% cobalt chromium, provides 30~35% metal coverage of the inner surface of the target vessel. This ratio is higher than that of conventional stents, which provide 6.5~9.5% of metal surface area coverage. Due to the low porosity (0.02-0.05 mm2) of this device, hemodynamic flow from the parent vessel cannot enter the aneurysm and sufficient scaffolding is provided to support neointimal regeneration of the neck. Preliminary clinical observations and experimental studies have shown that stents with low porosity may induce a high resistance to flow into the aneurysm, leading to flow stagnation in the sac and promotion of thrombosis and subsequent occlusion of the aneurysm.1)14)24) In a rabbit elastase aneurysm model, Kallmes et al.11)12) reported the occlusion rate of the PED and neointimal hyperplasia after stenting. Their second device (PED-2) provided approximately 35% of area coverage over the aneurysm neck. PED-2 achieved a superior occlusion rate (94% complete occlusion) compared with that of first-generation devices (53%) with 30% coverage. There were no cases of branch artery occlusion, either immediately after implantation or at follow-up. At six months, they harvested aneurysms and demonstrated that the tubular strut provides scaffolding for neointimal regeneration across the aneurysm neck. This regenerated endothelium over the device resulted in a contiguous layer between the proximal and distal portions of the parent artery in an in situ PED construct.
Lylyk et al.16) reported that in 53 patients with 63 aneurysms treated using PED, a 95% complete angiographic occlusion rate was achieved at 12 months of follow-up. Ninety four percent of the aneurysms arose from segmental lesions in which the vessel wall defect included more than 25% of the circumference of the parent artery, and 13% of the aneurysms were giant aneurysms. No major complications or recanalization occurred during the study period. Szikora et al.26) reported that among 19 wide-neck aneurysms in 18 patients treated with PED, the complete occlusion rate at six months of follow up was 94.4%. Nine cases were treated with a combination of PED and coils, however, all giant aneurysms (21.1%) were treated with PED alone. No difference in occlusion rate was observed between treatment using combined coil and that using PED alone. The morbidity and mortality rates were 5.6% each. Of particular interest, they showed that treatment with PED resulted in not only angiographic occlusion of the aneurysm but also reduction of the mass effect at the giant aneurysm. Saatci et al.22) reported the results of a large single-center series treated with PED; among 255 aneurysms in 191 patients, complete occlusion rates of 91.2% and 95.2% were achieved at six and 24 months, respectively, with a permanent morbidity rate of 1% and mortality rate of 0.5%. Of these, 11.8% were giant aneurysms, and at six months of follow up, 90% of aneurysms were completely occluded.
Although use of the PED has been limited in Korea, several studies have reported unfavorable complications. The rate of minor events was 9~16.7% and mortality was 0~6.4%.5)9)13)15)16)17)22)26)28) On systematic review, stroke due to thromboembolism was 3.2% and that of in stent stenosis more than 50% was 3.5%.19) The perforator occlusion, which is a main concern related to the PED, was reported as 2.5% by multiple applied devices, improper deployment, and resistance to antiplatelet medication.15)20)26)28) In addition, McAuliffe et al.17) experienced two cases of PED migration through the initial learning curve. After treatment with PED, intracranial hemorrhage of 0~6% was reported and possible causes were aneurysm rupture during the procedure, hemorrhagic transformation from ischemic tissue, inflammation of aneurysm wall, and foreign body's reaction.5)10)20)22)26)
In our experience using the PED for treatment of two giant intracranial aneurysms, the device flexibility was satisfactory with good stability. In both cases, the device was successfully apposed to the parent vessel wall without difficulty using standard microcatheters. Follow-up results confirmed that the PED affects the amount of blood flow into the sac and induces thrombosis in the sac.
References
1. Augsburger L, Farhat M, Reymond P, Fonck E, Kulcsar Z, Stergiopulos N, et al. Effect of flow diverter porosity on intraaneurysmal blood flow. Klin Neuroradiol. 2009; 8. 19(3):204–214. PMID: 19705075.

2. Barrow DL, Alleyne C. Natural history of giant intracranial aneurysms and indications for intervention. Clin Neurosurg. 1995; 42:214–244. PMID: 8846594.
3. Berenstein A, Song JK, Niimi Y, Namba K, Heran NS, Brisman JL, et al. Treatment of cerebral aneurysms with hydrogel-coated platinum coils (HydroCoil): early single-center experience. AJNR Am J Neuroradiol. 2006; 10. 27(9):1834–1840. PMID: 17032853.
4. Fiorella D, Albuquerque FC, Deshmukh VR, McDougall CG. Usefulness of the Neuroform stent for the treatment of cerebral aneurysms: results at initial (3-6-mo) follow-up. Neurosurgery. 2005; 6. 56(6):1191–1201. discussion 1201-2. PMID: 15918935.

5. Fischer S, Vajda Z, Aguilar Perez M, Schmid E, Hopf N, Bazner H, et al. Pipeline embolization device (PED) for neurovascular reconstruction: initial experience in the treatment of 101 intracranial aneurysms and dissections. Neuroradiology. 2012; 4. 54(4):369–382. PMID: 21881914.

6. Fujita K, Yamashita H, Masumura M, Nishizaki T, Tamaki N, Matsumoto S. [Natural history of giant intracranial aneurysms]. No Shinkei Geka. 1988; 3. 16(3):225–231. Japanese. PMID: 3287205.
7. Gruber A, Killer M, Bavinzski G, Richling B. Clinical and angiographic results of endosaccular coiling treatment of giant and very large intracranial aneurysms: a 7-year, single-center experience. Neurosurgery. 1999; 10. 45(4):793–803. discussion 803-4. PMID: 10515473.

8. Hauck EF, Welch BG, White JA, Replogle RE, Purdy PD, Pride LG, et al. Stent/coil treatment of very large and giant unruptured ophthalmic and cavernous aneurysms. Surg Neurol. 2009; 1. 71(1):19–24. discussion 24. PMID: 18423540.

9. Hirsch JA, Chandra RV, Leslie-Mazwi TM. Pipeline, aneurysms and the FDA. J Neurointerv Surg. 2012; 7. 4(4):314. author reply 314. PMID: 22442403.

10. Hu YC, Deshmukh VR, Albuquerque FC, Fiorella D, Nixon RR, Heck DV, et al. Histopathological assessment of fatal ipsilateral intraparenchymal hemorrhages after the treatment of supraclinoid aneurysms with the Pipeline Embolization Device. J Neurosurg. 2014; 2. 120(2):365–374. PMID: 24320006.

11. Kallmes DF, Ding YH, Dai D, Kadirvel R, Lewis DA, Cloft HJ. A new endoluminal, flow-disrupting device for treatment of saccular aneurysms. Stroke. 2007; 8. 38(8):2346–2352. PMID: 17615366.

12. Kallmes DF, Ding YH, Dai D, Kadirvel R, Lewis DA, Cloft HJ. A second-generation, endoluminal, flow-disrupting device for treatment of saccular aneurysms. AJNR Am J Neuroradiol. 2009; 6. 30(6):1153–1158. PMID: 19369609.

13. Kan P, Siddiqui AH, Veznedaroglu E, Liebman KM, Binning MJ, Dumont TM, et al. Early postmarket results after treatment of intracranial aneurysms with the pipeline embolization device: a U.S. multicenter experience. Neurosurgery. 2012; 12. 71(6):1080–1087. discussion 1087-8. PMID: 22948199.
14. Lieber BB, Stancampiano AP, Wakhloo AK. Alteration of hemodynamics in aneurysm models by stenting: influence of stent porosity. Ann Biomed Eng. 1997; May-Jun. 25(3):460–469. PMID: 9146801.
15. Lubicz B, Collignon L, Raphaeli G, De Witte O. Pipeline flow-diverter stent for endovascular treatment of intracranial aneurysms: preliminary experience in 20 patients with 27 aneurysms. World Neurosurg. 2011; Jul-Aug. 76(1-2):114–119. PMID: 21839962.

16. Lylyk P, Miranda C, Ceratto R, Ferrario A, Scrivano E, Luna HR, et al. Curative endovascular reconstruction of cerebral aneurysms with the pipeline embolization device: the Buenos Aires experience. Neurosurgery. 2009; 4. 64(4):632–642. discussion 642-3. PMID: 19349825.
17. McAuliffe W, Wycoco V, Rice H, Phatouros C, Singh TJ, Wenderoth J. Immediate and midterm results following treatment of unruptured intracranial aneurysms with the pipeline embolization device. AJNR Am J Neuroradiol. 2012; 1. 33(1):164–170. PMID: 21979492.

18. Murayama Y, Nien YL, Duckwiler G, Gobin YP, Jahan R, Frazee J, et al. Guglielmi detachable coil embolization of cerebral aneurysms: 11 years' experience. J Neurosurg. 2003; 5. 98(5):959–966. PMID: 12744354.

19. Murthy SB, Shah S, Venkatasubba Rao CP, Bershad EM, Suarez JI. Treatment of unruptured intracranial aneurysms with the pipeline embolization device. J Clin Neurosci. 2014; 1. 21(1):6–11. PMID: 24055205.

20. Nelson PK, Lylyk P, Szikora I, Wetzel SG, Wanke I, Fiorella D. The pipeline embolization device for the intracranial treatment of aneurysms trial. AJNR Am J Neuroradiol. 2011; 1. 32(1):34–40. PMID: 21148256.

21. Piotin M, Blanc R, Spelle L, Mounayer C, Piantino R, Schmidt PJ, et al. Stent-assisted coiling of intracranial aneurysms: clinical and angiographic results in 216 consecutive aneurysms. Stroke. 2010; 1. 41(1):110–115. PMID: 19959540.
22. Saatci I, Yavuz K, Ozer C, Geyik S, Cekirge HS. Treatment of intracranial aneurysms using the pipeline flow-diverter embolization device: a single-center experience with long-term follow-up results. AJNR Am J Neuroradiol. 2012; 9. 33(8):1436–1446. PMID: 22821921.

23. Sadasivan C, Lieber BB, Cesar L, Miskolczi L, Seong J, Wakhloo AK. Angiographic assessment of the performance of flow divertors to treat cerebral aneurysms. Conf Proc IEEE Eng Med Biol Soc. 2006; 1:3210–3213. PMID: 17946555.

24. Seshadhri S, Janiga G, Beuing O, Skalej M, Thevenin D. Impact of stents and flow diverters on hemodynamics in idealized aneurysm models. J Biomech Eng. 2011; 7. 133(7):071005. PMID: 21823744.

25. Sluzewski M, Menovsky T, van Rooij WJ, Wijnalda D. Coiling of very large or giant cerebral aneurysms: long-term clinical and serial angiographic results. AJNR Am J Neuroradiol. 2003; 2. 24(2):257–262. PMID: 12591644.
26. Szikora I, Berentei Z, Kulcsar Z, Marosfoi M, Vajda ZS, Lee W, et al. Treatment of intracranial aneurysms by functional reconstruction of the parent artery: the Budapest experience with the pipeline embolization device. AJNR Am J Neuroradiol. 2010; 6. 31(6):1139–1147. PMID: 20150304.

27. Wiebers DO, Whisnant JP, Huston J 3rd, Meissner I, Brown RD Jr, Piepgras DG, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003; 7. 362(9378):103–110. PMID: 12867109.

28. Yu SC, Kwok CK, Cheng PW, Chan KY, Lau SS, Lui WM, et al. Intracranial aneurysms: midterm outcome of pipeline embolization device-a prospective study in 143 patients with 178 aneurysms. Radiology. 2012; 12. 265(3):893–901. PMID: 22996749.

Fig. 1
A giant aneurysm located at the C3-C4 portion of the left internal carotid artery. Initial T2-weighted MRI (A), cerebral angiography (B), SPECT (C), after deployment of PED (D and E), CTA three months later (F), angiography six months later (G and H), CTA nine months later showing sac shrinkage (I). MRI = magnetic resonance imaging; SPECT = single photon emission computed tomography; PED = pipeline embolization device; CTA = computerized tomography angiography
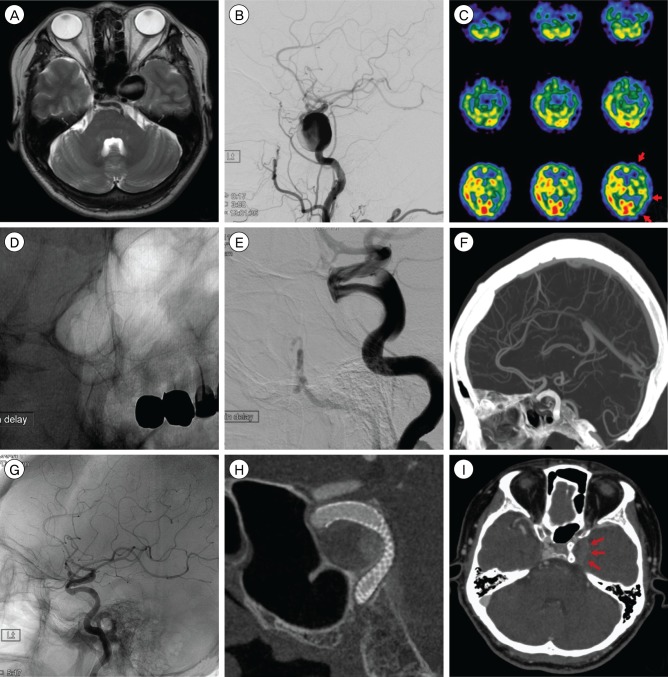
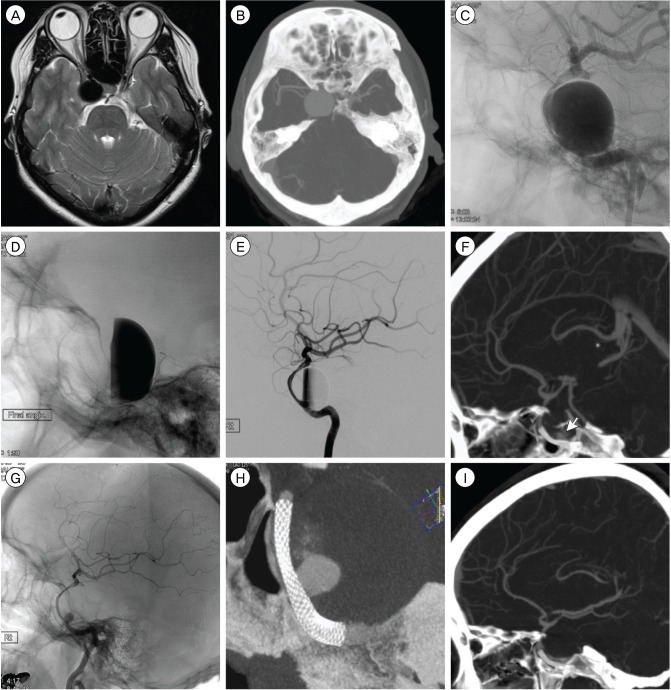
Fig. 2
A giant aneurysm located at the C4-C5 portion of the right internal carotid artery. Initial T2-weighted MRI (A), follow-up CTA (B), cerebral angiography (C), after deployment of PED (D and E), CTA three months later with small filling portion (F), angiography six months later (G) and (H), CTA nine months later without remnant sac (I).
MRI = magnetic resonance imaging; PED = pipeline embolization device; CTA = computerized tomography angiography




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download