Abstract
Objective
Arterial stiffness is a common change associated with aging and can be evaluated by measuring pulse wave velocity (PWV) between sites in the arterial tree, with the stiffer artery having the higher PWV. Arterial stiffness is associated with the risk of stroke in the general population and of fatal stroke in hypertensive patients. This study is to clarify whether PWV value predicts functional outcome of acute ischemic stroke.
Methods
One hundred patients were enrolled with a diagnosis of acute ischemic stroke and categorized into two groups: large-artery atherosclerosis (LAAS) or small vessel disease (SVD) subtype of Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification. Each group was divided into two sub-groups based on the functional outcome of acute ischemic stroke, indicated by modified Rankin Scale (mRS) at discharge. Poor functional outcome group was defined as a mRS ≥ 3 at discharge. Student's t-test or Mann-Whitney U-test were used to compare maximal brachial-ankle PWV (baPWV) values.
Results
Twenty-four patients whose state was inadequate to assess baPWV or mRS were excluded. There were 38 patients with good functional outcome (mRS < 3) and 38 patients with poor functional outcome (mRS ≥ 3). The baPWV values were significantly higher in patients with poor outcome (2,070.05 ± 518.37 cm/s) compared with those with good outcome (1,838.63 ± 436.65) (p = 0.039). In patients with SVD subtype, there was a significant difference of baPWV values between groups (2,163.18 ± 412.71 vs. 1,789.80 ± 421.91, p = 0.022), while there was no significant difference of baPWV among patients with LAAS subtype (2,071.76 ± 618.42 vs. 1,878.00 ± 365.35, p = 0.579).
Arterial stiffness is a common change associated with aging.28) Vascular stiffening develops from a complex interplay between stable and dynamic changes involving structural and cellular elements of the vessel wall. These alterations of arterial stiffening are closely related to a multitude of potentially interacting factors including atherosclerosis, hemodynamic forces, hormonal milieu, endothelial dysfunction, oxidative stress, inflammation, and vascular calcification.17)33)38)39)41) The role of biochemical changes in arterial stiffness in acute ischemic stroke is not known. Current research on the relation between arterial stiffness and cardiovascular disease demonstrates that arterial stiffness contributes to cardiovascular diseases in older individual.29)
Pulse wave velocity (PWV) is a simple, non-invasive indicator of arterial stiffness and has been researched extensively to confirm its value.15) PWV value is usually measured as the carotid-femoral PWV (cfPWV) or the brachial-ankle PWV (baPWV).3)17)32)39) In particular, baPWV is ideal for large scale population studies because it is simpler than cfPWV.19)30)40)
Increased PWV is associated with higher risk and mortality of cardiovascular events such as coronary heart disease and stroke.13)18) Increased PWV is also associated with fatal stroke.14) Among stroke patients, small vessel disease (SVD) subtype categorized by Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification had the highest PWV value.32) Recent studies have demonstrated that baPWV is independently associated with the risk and severity of cerebral ischemic SVD.10)11) However, no study has yet evaluated the association between arterial stiffness and functional outcome in patients with ischemic stroke.
Our study hypothesizes that the patient with poor functional outcome has a higher PWV value. This study is to clarify whether PWV value predicts functional outcome of acute ischemic stroke. Furthermore, we investigated whether there is a difference between large-artery atherosclerosis (LAAS) and SVD subtypes categorized by TOAST classification.
This retrospective study included consecutive acute ischemic stroke patients who were admitted to the Gachon University Gil Medical Center between August 2011 and October 2012. A diagnosis of acute ischemic stroke was defined by focal neurological signs or symptoms that continued for > 24 hours thought to be of vascular origin. The final diagnosis of ischemic stroke was confirmed by magnetic resonance imaging (MRI). The inclusion criteria were LAAS and SVD subtypes categorized by TOAST classification. Patients with spinal cord disease, neuromuscular disease and trauma or pain were excluded because of their effect on functional outcome regardless of stroke. Demographic and baseline clinical data were carefully investigated. During admission, all patients were evaluated with complete blood count, serum lipid profile, serologic test, electrocardiography (ECG) and echocardiography. Low-density lipoprotein (LDL) was calculated according to Friedewald equation. Every patient's height and weight were measured, and the body mass index (BMI) calculated by dividing weight by height squared. All patients underwent stroke imaging study including brain computed tomography (CT), MRI and MR angiography (MRA). We measured the common carotid artery intima-media thickness (IMT) by ultrasonography on both the left and right sides, and the higher IMT value was chosen for analyses. The type of acute ischemic stroke was classified by the TOAST classification.1) The diagnosis of LAAS requires either significant (> 50%) stenosis or occlusion of a major brain artery of branch cortical artery, relevant to the infarct lesion. SVD is diagnosed when a patient has the traditional clinical lacunar syndromes and shows an either normal CT/MRI examination or small infarct lesion (< 15 mm) in the perforating artery territory, but no evidence of cerebral cortical dysfunction and of LAAS and cardioembolism. At discharge, the functional outcome of ischemic stroke was evaluated with the modified Rankin scale (mRS). Good functional outcome was defined as mRS score of 0-2 at hospital discharge. Severity of neurological deficit was determined by the National Institute of Health Stroke Scale (NIHSS) at both hospital admission and discharge. The study protocol was approved (H-1108-026-019) by the Institutional Review Board of Gil Hospital, and all participants were given with written informed consent.
Cardiovascular risk factors were evaluated by the following criteria. Hypertension was defined as new diagnosis during admission according to the World Health Organization/International Society of Hypertension guidelines or as present if subjects had been previously diagnosed and/or were receiving antihypertensive medication. Diabetes mellitus was specified as fasting serum glucose ≥ 126 mg/dl, non-fasting serum glucose ≥ 200 mg/dl, hemoglobin A1c level ≥ 6.5% or a history of insulin therapy or oral hypoglycemic drugs. Hypercholesterolemia was defined as the presence of total cholesterol blood levels ≥ 200 mg/dl or use of statins. History of previous cerebrovascular disease (transient ischemic attack (TIA) or ischemic stroke) and coronary artery disease (myocardial infarction, angina pectoris or angioplasty), and current smoking habit were recorded.
baPWV was measured using an automatic device (Colin VP 1000, Omron co., Kyoto, Japan). The distance between the brachium and the ankle for baPWV was calculated by the height of the patient. PWV was calculated as the distance between the brachial and the ankle divided by the time delay between the two arterial points, expressed as centimeters per second. After examinations were performed on both the left and right sides, the larger value was used for further analyses.
SPSS statistics 18.0 program (SPSS Inc., Chicago, IL, United States) was used for statistical analysis. Continuous data such as age, BMI and baPWV are expressed as the mean ± SD and were compared by using Student's t-tests or Mann-Whitney U-tests. Categorical data are expressed as frequencies and percentages and were compared by using chi-square tests. A two-tailed p value < 0.05 are considered statically significant.
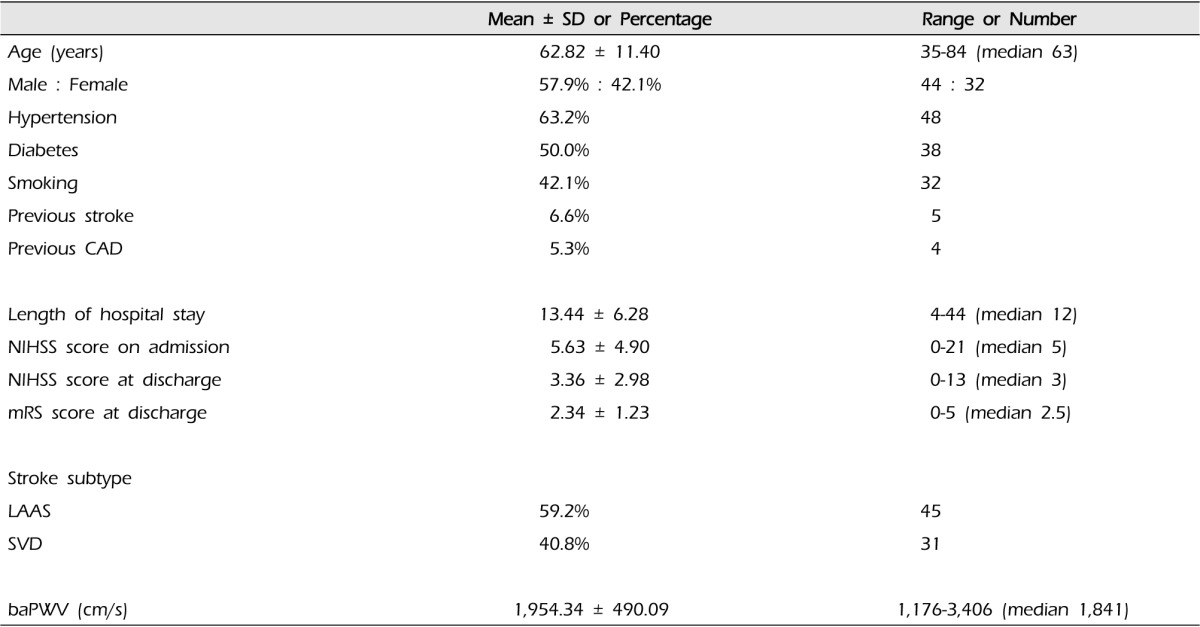
We recruited 100 acute ischemic stroke patients, of whom 89 had technically adequate baPWV measurements. Of these 89 patients, 76 had adequate assessments of functional outcome with mRS at discharge. We report the findings of these 76 ischemic stroke patients.
The demographic data of the 76 ischemic stroke patients were median age of 62.82 years (range 35-84 years), 57.9% were male patients, 63.2% hypertensive patients, 50.0% diabetic patients and 42.1% smokers. There were five patients with previous stroke and four patients with history of coronary artery disease. The mean of NIHSS score at admission was 5.63 ± 4.90. Regarding the functional outcome at discharge, the mean of mRS score was 2.34 ± 1.23. According to the TOAST classification, the etiology of stroke was LAAS in 45 (59.2%) patients whereas 31 patients (40.8%) were classified as SVD (Table 1).
We divided the 76 patients into two groups based on mRS score at discharge. There were 38 patients with good functional outcome (mRS < 3) and 38 patients with poor functional outcome (mRS ≥ 3). Patients with poor functional outcome had a higher prevalence of diabetes than those with good functional outcome (65.8% vs. 34.2%, p = 0.006) (Table 2). There was no difference in functional outcome by age, sex, hypertension, hypercholesterolemia, smoking, history of stroke and coronary artery disease, BMI, serum cholesterol, hemoglobin A1c, blood pressure, stroke subtype and IMT. Arterial stiffness measured by baPWV was significantly higher in patients with poor outcome compared to with those with good outcome (2,070.05 ± 518.37 vs. 1,838.63 ± 436.65, p = 0.039). The difference in the mean NIHSS score at admission and discharge between good and poor functional outcome groups was statistically significant (3.24 ± 3.58 vs. 8.03 ± 4.91, p < 0.001 and 1.46 ± 1.33 vs. 5.27 ± 2.96, p < 0.001).
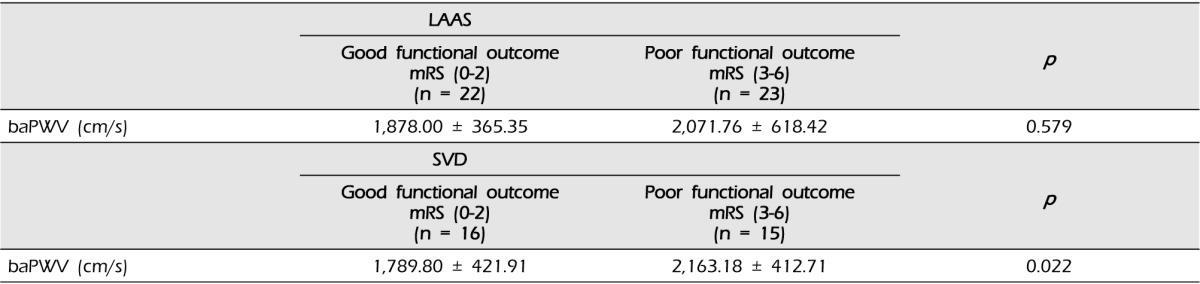
In LAAS subtype, there were 22 patients with good functional outcome and 23 patients with poor functional outcome. The difference in the mean of baPWV between good outcome and poor outcome was not statistically significant (1,878.00 ± 365.35 vs. 2,071.76 ± 618.42, p = 0.579). Among the subjects with SVD subtype, 16 patients had good functional outcome and 15 patients had poor functional outcome. There was a significant difference in baPWV values between good and poor outcome groups (1,789.80 ± 421.91 vs. 2,163.18 ± 412.71, p = 0.022) (Table 3).
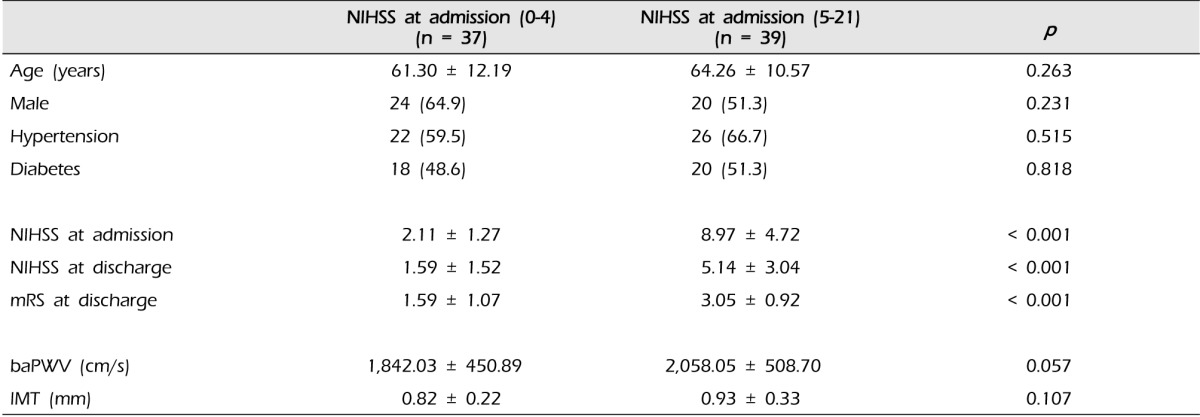
The 76 patients were divided into two outcome variables according to initial stroke severity measured by NIHSS score at admission. There were 37 patients with NIHSS score ≤ 4 at admission and 39 patients with NIHSS score ≥ 5 at admission. There were no differences by age, sex, hypertension, diabetes and IMT between groups. The difference in the mean mRS at discharge was statistically significant (NIHSS sore < 4, 1.59 ± 1.07 vs. NIHSS score ≥ 5, 3.05 ± 0.92, p < 0.001). The difference between baPWV and initial severity was not significant. However, there was a tendency for the patients with severe symptoms to have a higher level of baPWV (Table 4).
We describe a novel relationship between arterial stiffness indicated by baPWV and functional outcome in patients with acute ischemic stroke, evidenced by patients with poor functional outcome having higher baPWV value. However, the novel relationship did not represent an independent association between baPWV and functional outcome of stroke. Other factors such as diabetes mellitus and initial stroke severity, could have acted as confounding variables.
Aortic stiffness measured by PWV is associated with risk of stroke in general population and of fatal stroke in hypertensive patients.14)37) This study suggests that baPWV is associated with clinical outcome after acute ischemic stroke. The mechanisms underlying this relationship are unclear.
Arterial stiffness is associated with a number of deleterious vascular conditions.2)33)41) One of the inevitable conditions of an aging vascular system is the presence of atherosclerosis.33)39) In addition, intimal change thickens the aortic wall even without atherosclerotic disease.21)34) The elastin fibers are depleted and fragmented by the aging process,9)24)27) leadings to vessel wall toughening and thus increasing the central pressure.9)22) This, in turn, prompts an inadequate increase in systolic blood pressure and relative decrease in diastolic blood pressure. As a result, pulse pressure increases with arterial stiffening.5)25)36) Stiffer arteries cause high pulsatile pressure and flow to be transmitted to small vessels of distal organs during systole, eventually leading to microvascular damage in the brain.8)25) Hence, indirect evidence supports the relationship between arterial stiffness and functional outcome of stroke.
The present study shows the importance of finding prognostic predictors in patients with acute ischemic stroke. Stroke is devastating to both patient and family due to its high mortality, severe sequelae, and significant financial burden. Thus, the medical sequeles, such as death, recurrence, or functional recovery after the event, are of great concern to both the family of the patient and to the medical staff. To plan an appropriate level of treatment and rehabilitation of stroke patients, medical staffs are asked to precisely anticipate the degree of disabilities and recovery of normal function. Therefore, devising a simple test for stroke patients will help to establish the appropriate treatment plan. Our study found an association between high levels of baPWV and worse functional outcome, suggesting that baPWV could be safely used as a prognostic factor for anticipating patients' prognosis.
Recent studies demonstrated that cfPWV is associated with clinical outcome after ischemic stroke. One recent study compared cfPWV with mRS that was measured within 90 days from the onset of stroke, and another study compared NIHSS measured at the time of discharge.6)7) baPWV was significantly and positively associated with cfPWV. Both PWV measures were similarly associated with cardiovascular disease risk factors and clinical events.13) This is strong evidence supporting the relationship of baPWV to functional outcome of the stroke.
cfPWV represents the gold standard among the PWV parameters, but this measurement cannot be performed easily as a screen for older patients.39) On the other hand, baPWV uses a pressure cuff wrapped on the brachium and ankle, and is simpler than other noninvasive automatic devices. It has a potential application for screening arterial stiffness in a larger population because of its simplicity and short sampling time.19)40)
Carotid IMT is a non-invasive measure to assess sub-clinical atherosclerotic change in carotid artery and is predictive of all-cause and cardiovascular mortality in elderly people.20)35) Generally, IMT measurements > 1.1 mm are accepted as abnormal.23) Several studies suggest a significant association between carotid IMT and PWV.12)16)31) In the present study, there is no relationship between IMT and functional outcome after acute ischemic stroke.
In our study, functional outcome and arterial stiffness measured by baPWV are associated in the SVD subtype; but not in the LAAS subtype. Increased baPWV is associated with risk of small vessel disease in elderly patients with hypertension, with severity of cerebral SVD, the number of lacunar infarcts and silent brain infarct.10)11)26) Furthermore, arterial stiffness is associated with renal failure arising from the microvascular damage.4)25)
Microvascular features of the kidney and brain have similar continuous and passive perfusion at high-volume flow throughout systole and diastole.25) This indirectly supports an association of increased baPWV with SVD subtype of stroke. Further investigation of association between arterial stiffness and SVD is required because the causes of these findings are not yet clearly known.
Additional analysis of the relation between arterial stiffness and initial severity showed no significant difference. However, there was a trend that the patients with higher levels of arterial stiffness had more severe symptoms when they presented at the hospital.
Initial severity of stroke is a strong predictor of long-term outcome in patients with ischemic stroke and thus, further research is required to assess the relation between arterial stiffness and initial severity, could answer the question of whether arterial stiffness itself directly has an effect on brain damage or impedes the recovery of brain function.
Our study had several limitations. The first is the small sample size, limiting generalizability of the result. A large population study is needed to confirm the results. Second, the study did not include very severe patients because it was difficult to get their permission for the study due to their adverse medical condition, and this led to biased selection. Therefore, we advise cautions in interesting the findings beyond this group. Third, our study has a wide range of distribution of patients' hospitalization time and variable time of mRS measurement. It is more accurate to compare data from a fixed day from stroke onset. However, we believe it does not significantly influence the results. Finally, our study did not control the confounding variables. Future study might need to control confounding variables.
Arterial stiffness indicated by baPWV is associated with the functional outcome of acute ischemic stroke. Measurement of arterial stiffness could be a useful marker for assessing early functional outcome in patients with acute ischemic stroke especially whose TOAST classification is confirmed as SVD.
References
1. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993; 1. 24(1):35–41. PMID: 7678184.

2. Blacher J, Asmar R, Djane S, London GM, Safar ME. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension. 1999; 5. 33(5):1111–1117. PMID: 10334796.

3. De Silva DA, Woon FP, Gan HY, Chen CP, Chang HM, Koh TH, et al. Arterial stiffness is associated with intracranial large artery disease among ethnic Chinese and South Asian ischemic stroke patients. J hypertens. 2009; 7. 27(7):1453–1458. PMID: 19357532.

4. DeLoach SS, Townsend RR. Vascular stiffness: Its measurement and significance for epidemiologic and outcome studies. Clin J Am Soc Nephrol. 2008; 1. 3(1):184–192. PMID: 18178784.

5. Franklin SS, Gustin W 4th, Wong ND, Larson MG, Weber MA, Kannel WB, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997; 7. 96(1):308–315. PMID: 9236450.
6. Gasecki D, Rojek A, Kwarciany M, Kowalczyk K, Boutouyrie P, Nyka W, et al. Pulse wave velocity is associated with early clinical outcome after ischemic stroke. Atherosclerosis. 2012; 12. 225(2):348–352. PMID: 23083680.

7. Gasecki D, Rojek A, Kwarciany M, Kubach M, Boutouyrie P, Nyka W, et al. Aortic stiffness predicts functional outcome in patients after ischemic stroke. Stroke. 2012; 2. 43(2):543–544. PMID: 22076001.

8. Izzo JL Jr. Arterial stiffness and the systolic hypertension syndrome. Curr Opin Cardiol. 2004; 7. 19(4):341–352. PMID: 15218394.

9. Kass DA. Age-related changes in venticular-arterial coupling: Pathophysiologic implications. Heart Fail Rev. 2002; 1. 7(1):51–62. PMID: 11790922.
10. Kim DH, Choi JH, Moon JS, Kim HJ, Cha JK. Association between the severity of cerebral small vessel disease, pulsatility of cerebral arteries, and brachial ankle pulse wave velocity in patients with lacunar infarction. Eur Neurol. 2010; 64(4):247–252. PMID: 20838053.

11. Kim DH, Kim J, Kim JM, Lee AY. Increased brachial-ankle pulse wave velocity is independently associated with risk of cerebral ischemic small vessel disease in elderly hypertensive patients. Clin Neurol Neurosurg. 2008; 6. 110(6):599–604. PMID: 18471955.

12. Kobayashi K, Akishita M, Yu W, Hashimoto M, Ohni M, Toba K. Interrelationship between non-invasive measurements of atherosclerosis: Flow-mediated dilation of brachial artery, carotid intima-media thickness and pulse wave velocity. Atherosclerosis. 2004; 3. 173(1):13–18. PMID: 15177119.

13. Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001; 5. 37(5):1236–1241. PMID: 11358934.

14. Laurent S, Katsahian S, Fassot C, Tropeano AI, Gautier I, Laloux B, et al. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke. 2003; 5. 34(5):1203–1206. PMID: 12677025.

15. Lehmann ED. Clinical value of aortic pulse-wave velocity measurement. Lancet. 1999; 8. 354(9178):528–529. PMID: 10470691.

16. Mackey RH, Sutton-Tyrrell K, Vaitkevicius PV, Sakkinen PA, Lyles MF, Spurgeon HA, et al. Correlates of aortic stiffness in elderly individuals: A subgroup of the Cardiovascular Health Study. Am J Hypertens. 2002; 1. 15(1 Pt 1):16–23. PMID: 11824854.

17. Matsumoto M, Inoue K, Moriki A. Associations of brachial-ankle pulse wave velocity and carotid atherosclerotic lesions with silent cerebral lesions. Hypertens Res. 2007; 9. 30(9):767–773. PMID: 18037768.

18. Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, et al. Arterial stiffness and risk of coronary heart disease and stroke: The Rotterdam Study. Circulation. 2006; 2. 113(5):657–663. PMID: 16461838.
19. Munakata M, Ito N, Nunokawa T, Yoshinaga K. Utility of automated brachial ankle pulse wave velocity measurements in hypertensive patients. Am J Hypertens. 2003; 8. 16(8):653–657. PMID: 12878371.

20. Murakami S, Otsuka K, Hotta N, Yamanaka G, Kubo Y, Matsuoka O, et al. Common carotid intima-media thickness is predictive of all-cause and cardiovascular mortality in elderly community-dwelling people: Longitudinal Investigation for the Longevity and Aging in Hokkaido County (LILAC) study. Biomed Pharmacother. 2005; 10. 59(Suppl 1):S49–S53. PMID: 16275507.

21. Nagai Y, Metter EJ, Fleg JL. Increased carotid artery intimal-medial thickness: Risk factor for exercise-induced myocardial ischemia in asymptomatic older individuals. Vasc Med. 1999; 4(3):181–186. PMID: 10512598.

22. Nichols WW. Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. Am J Hypertens. 2005; 1. 18(1 Pt 2):3S–10S. PMID: 15683725.

23. O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK Jr. Cardiovascular Health Study Collaborative Research Group. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. N Engl J Med. 1999; 1. 340(1):14–22. PMID: 9878640.
24. O'Rourke MF, Nichols WW. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension. 2005; 4. 45(4):652–658. PMID: 15699456.
25. O'Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: Cause and logic of therapy. Hypertension. 2005; 7. 46(1):200–204. PMID: 15911742.
26. Saji N, Kimura K, Shimizu H, Kita Y. Association between silent brain infarct and arterial stiffness indicated by brachial-ankle pulse wave velocity. Intern Med. 2012; 51(9):1003–1008. PMID: 22576377.

27. Santhanam L, Christianson DW, Nyhan D, Berkowitz DE. Arginase and vascular aging. J Appl Physiol (1985). 2008; 11. 105(5):1632–1642. PMID: 18719233.

28. Shirwany NA, Zou MH. Arterial stiffness: A brief review. Acta Pharmacol Sin. 2010; 10. 31(10):1267–1276. PMID: 20802505.

29. Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005; 6. 111(25):3384–3390. PMID: 15967850.

30. Tanaka H, Munakata M, Kawano Y, Ohishi M, Shoji T, Sugawara J, et al. Comparison between carotid-femoral and brachial-ankle pulse wave velocity as measures of arterial stiffness. J Hypertens. 2009; 10. 27(10):2022–2027. PMID: 19550355.

31. Taniwaki H, Kawagishi T, Emoto M, Shoji T, Kanda H, Maekawa K, et al. Correlation between the intima-media thickness of the carotid artery and aortic pulse-wave velocity in patients with type 2 diabetes. Vessel wall properties in type 2 diabetes. Diabetes Care. 1999; 11. 22(11):1851–1857. PMID: 10546019.

32. Tuttolomondo A, Di Sciacca R, Di Raimondo D, Serio A, D'Aguanno G, Pinto A, et al. Arterial stiffness indexes in acute ischemic stroke: Relationship with stroke subtype. Atherosclerosis. 2010; 7. 211(1):187–194. PMID: 20226464.

33. van Popele NM, Grobbee DE, Bots ML, Asmar R, Topouchian J, Reneman RS, et al. Association between arterial stiffness and atherosclerosis: The Rotterdam Study. Stroke. 2001; 2. 32(2):454–460. PMID: 11157182.
34. Virmani R, Avolio AP, Mergner WJ, Robinowitz M, Herderick EE, Cornhill JF, et al. Effect of aging on aortic morphology in populations with high and low prevalence of hypertension and atherosclerosis. Comparison between occidental and Chinese communities. Am J Pathol. 1991; 11. 139(5):1119–1129. PMID: 1951629.
35. Wardlaw JM. Carotid imaging for secondary stroke prevention in routine practice. Int J Stroke. 2008; 2. 3(1):20–32. PMID: 18705912.

36. Wilkinson IB, Franklin SS, Hall IR, Tyrrell S, Cockcroft JR. Pressure amplification explains why pulse pressure is unrelated to risk in young subjects. Hypertension. 2001; 12. 38(6):1461–1466. PMID: 11751736.

37. Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, et al. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006; 2. 113(5):664–670. PMID: 16461839.

38. Wolinsky H, Glagov S. Structural basis for the static mechanical properties of the aortic media. Circ Res. 1964; 5. 14:400–413. PMID: 14156860.

39. Yamashina A, Tomiyama H, Arai T, Hirose K, Koji Y, Hirayama Y, et al. Brachial-ankle pulse wave velocity as a marker of atherosclerotic vascular damage and cardiovascular risk. Hypertens Res. 2003; 8. 26(8):615–622. PMID: 14567500.

40. Yamashina A, Tomiyama H, Takeda K, Tsuda H, Arai T, Hirose K, et al. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res. 2002; 5. 25(3):359–364. PMID: 12135313.

41. Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005; 5. 25(5):932–943. PMID: 15731494.

Table 2
Demographic and risk factors in ischemic stroke patients by functional outcome
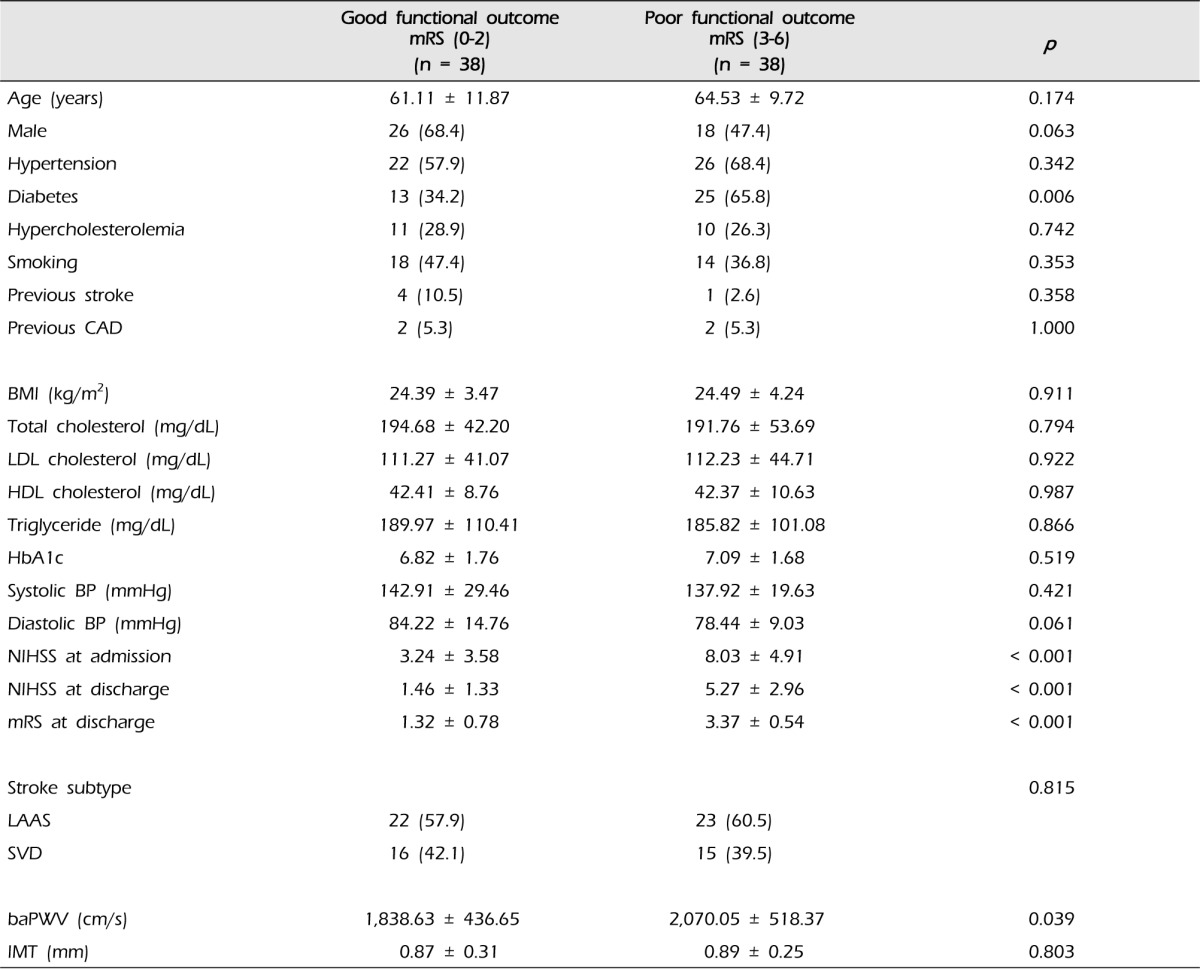
mRS = modified Rankin scale; CAD = coronary artery disease; LDL = low density lipoprotein; HDL = high density lipoprotein; BP = blood pressure; NIHSS = National Institute of Health Stroke Scale; LAAS = large-artery atherosclerosis; SVD = small vessel disease; baPWV = brachial-ankle pulse wave velocity; IMT = intima-media thickness.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download