Abstract
We experienced a patient with a ruptured dissecting aneurysm of the vertebral artery who was treated by trapping of the lesion using Guglielmi detachable coils (GDCs) with micro-tornado® coils (MTCs). An 80-year-old male was transferred with a ruptured left vertebral artery dissecting aneurysm (VADA). The dissected portion of the vertebral artery was effectively trapped using GDCs and MTCs. The MTCs used for neurointervention were comprised of various types of coils and we successfully placed them into the parent artery of the dissected segment. The author suggests that this case demonstrates the usefulness of endovascular coil trapping of VADAs using MTCs in achievement of embolization.
Vertebral artery dissecting aneurysms (VADAs) are diagnosed as a reason for subarachnoid hemorrhage (SAH).14) Once ruptured and causing SAH, the percentage of re-bleeding of these lesions is up to 30% and is associated with a high rate of mortality.1)18) Thus, early treatment is strongly recommended. The most generally recognized treatment tool is complete occlusion of the dissected lesion by open surgery or endovascular procedures. Because the surgical approach is associated with high incidence of treatment-associated mortality and morbidity, endovascular procedures are favored in treatment of dissecting aneurysms of the vertebral artery (VA).8)15)16) In endovascular procedures, proximal occlusion of the parent artery and embolization of the dissected segment using Guglielmi detachable coils (GDCs) has proven to be an optimal method for treatment of dissecting aneurysms.5) Using flow diverter devices, such as the Pipeline embolization device and Silk stents is a new concept for endovascular treatment of intracranial aneurysms.11) Various forms of coils have been approved for use in endovascular procedures. Of the available embolization coils, DCs are especially applied to specific cerebral lesions, namely intracranial aneurysms. However, in order to achieve the trapping of lesions, fiber-coated platinum coils are generally preferred over DCs because of their convenience of use and thrombogenic characteristics.7)17)
In this report, we describe the treatment of a patient using a combination of GDC and micro-tornado® coil (MTC) embolization in trapping of the VADA.
An 80-year-old male was transferred to our emergency room, with mental deterioration after posterior neck pain and sudden severe headache (Hunt and Hess grade IV). Computed tomography (CT) of the brain showed SAH and intraventricular hemorrhages that were more predominant in cisterns around the medulla and pons than in the basal cistern (Fisher grade IV) (Fig. 1). CT angiography showed a dissecting aneurysm arising from the left VA. A CT source image also showed a pseudoaneurysm and intimal flap, diagnosed with a VADA (Fig. 2).
A vertebral angiogram showed a left VADA measuring 5 × 8 mm in size, which showed irregular narrowing and a dilated portion. The left posterior inferior cerebellar artery (PICA) was visualized at the distal portion of the dissected segment of the left VA (Fig. 3). Endovascular coil trapping of the dissected segment was performed just after diagnostic angiography using six GDCs and two MTCs (Fig. 4).
In this case, the sheath was placed in the right femoral artery. A 6 Fr Envoy catheter (Cordis Endovascular Systems, Bridgewater, NJ) was placed in the left subclavian artery near the origin portion of the VA. After placement of the guiding catheter, the patient received a bolus injection of heparin (2000 U) and control-activated clotting times were determined hourly. We planned VA occlusion by trapping on both sides of the lesion, using the double microcatheter technique.9) First, an Excelsior™ SL-10 microcatheter (Boston Scientific Corp, Natick, MA) was manipulated with the tip curved 45° and advanced into the distal left VA using a Synchro®-14 neuro guidewire (Stryker Neurovascular, Kalamazoo, MI). The microcatheter was advanced slowly into the lesion up to the distal end of the dissected segment of the aneurysm. The attempt to advance the other microcatheter into the proximal end of the DA failed because the stenotic lesion of the proximal portion of the DA was too small to pass to the other microcatheter. The right VA origin portion was severely tortuous; thus, we failed to apply the double microcatheter technique. Instead, a Rebar™-18 microcatheter (ev3 Endovascular, Plymouth, MN) was advanced into the proximal portion of the stenotic lesion using a microguidewire. Multiple GDCs, totaling 42 cm in length, were placed at the dilated segment and two MTCs (Tornado® Embolization Microcoil™, Cook incorporated, Bloomington, IN, proximal end diameter 3 mm × distal end diameter 2 mm) were then deployed (Fig. 4). The size of MTCs was selected to match the diameter of the trapped segment. Before detachment of the GDCs, repeat angiography was performed in order to check coil placement at the affected site. After deployment of the MTCs, postoperative angiography showed complete occlusion of the dissecting aneurysm, while the left PICA was sacrificed. The patient was transported to the neurological intensive care unit with no occurrence of remarkable events. An magnetic resonance (MR) angiography performed one day after the procedure showed complete occlusion of the left VADA with appropriate flow to the basilar artery via the right VA and no regrowth or residual portion of the aneurysm (Fig. 5A, B).
Fortunately, the patient regained consciousness. A follow-up MR image showed embolic ischemic lesions in the left cerebellum (Fig. 5C), however, no neurological deficit was detected and no re-bleeding from the aneurysm was observed.
VADAs have a very high incidence of early re-bleeding and re-bleeding is associated with a poor prognosis and negative clinical outcome.14) Considering the features of VADAs and high risk, the most effective treatment is parent vessel occlusion. Either surgical clipping or endovascular balloon or coil occlusion can be used.6) Treatment of VADAs with proximal occlusion of the parent artery by means of endovascular procedures or surgical clipping is currently widely used. However, these procedures are not always very effective, yet they have been shown to prevent re-rupture.10) Open surgical procedures include clipping, wrapping, or trapping of the segment of dissecting aneurysm. However, surgical approaches generally pose a serious risk of morbidity in order to achieve their purpose. While endovascular treatments consist of deconstructive procedures and reconstructive procedures, deconstructive procedures typically include a proximal obliteration performed using GDCs, or obliteration at the dissecting segment performed using coils. On the other hand, reconstructive procedures include an aneurysm endovascular coil embolization or the combined procedure in which a stent and coil are used.12) Several researchers have recently shown that even a deconstructive approach performed by endovascular treatments does not perfectly eliminate the risk of re-bleeding from VADA.10) However, endovascular treatment for obliteration of the dissected segment, including both the dissecting aneurysm and parent artery, should be considered as the optimal treatment option in patients who are willing to sacrifice the parent artery and trap the dissected segment.13)
Improvements have been made in various forms of coils for use in endovascular procedures.3) Embolization materials are being rapidly developed; indications for endovascular treatments are more varied and widely extended. Of the embolization coils, DCs are applied to specific cerebral lesions, namely intracranial aneurysms.3) The convenience of the DCs is that they can be re-captured if they are not suitable for the location of the lesion. However, DCs are expensive and endovascular treatment using DCs has low cost efficacy. For achievement of the trapping of arteries or veins, fiber-coated platinum coils are generally preferred over DCs because of the convenience of using thrombogenic characteristics.7)17) In addition, the cost of fiber-coated coils is less than 10% of that of GDCs.4) The MTC is a platinum coil with synthetic fibers (Fig. 6A) used for embolization of selective vessel supply to vascular lesions; A longer-length coil with a tornado-like cone shape maximizes coil exposure to the cross-section of the lumen for interruption of blood flow and is available for delivery with 0.018, 0.035 and 0.038 inch end-hole catheters. Compared to un-coated coils, the synthetic fiber-coated coils can rise to activate thrombogenesis. Advancements in microcatheters have contributed significantly to improvement of endovascular procedures and have led to a need for fiber-coated coils of 0.018 inch in diameter. MTCs are a 0.018 inch fiber-coated platinum microcoil with a proximal end diameter ranging from 3 to 10 mm and distal end diameter ranging from 2 to 4 mm. MTCs were deployed to the vascular lesion for obliteration, allowing for smooth passage of microcatheters with a 0.018 inch inner diameter. Complete occlusion of the diseased portion was performed and no remarkable negative effect appeared in relation to MTC embolization. Hayashi et al.4) have reported several cases of parent artery occlusion of intracerebral aneurysms using Micro-Nester coils (similar to MTCs) (Fig. 6B). However, for complete obliteration, tight packing must be attained because recanalization may occur. Some authors have reported that the measured rate of recanalization is up to 10%,4) and the high flow or high pressure potential of a major artery is considered a cause of recanalization.4) As we experienced minimal neurologic deficits after trapping of the VA, occlusion of the PICA does not always lead to neurologic complications because of the presence of rich pial anastomoses.2)
Although our experience is limited to a few cases, the goal of our research was to understand our experience with MTCs in treatment of VADA and to assess the usefulness of MTC embolization.
References
1. Aoki N, Sakai T. Rebleeding from intracranial dissecting aneurysm in the vertebral artery. Stroke. 1990; 11. 21(11):1628–1631. PMID: 2237959.

2. Byrne JV, Beltechi R, Yarnold JA, Birks J, Kamran M. Early experience in the treatment of intra-cranial aneurysms by endovascular flow diversion: a multicentre prospective study. PLoS One. 2010; 9. 5(9):e12492. PMID: 20824070.

3. Guglielmi G, Vinuela F, Sepetka I, Macellari V. Electrothrombosis of saccular aneurysms via endovascular approach. Part 1: Electrochemical basis, technique and experimental results. J Neurosurg. 1991; 7. 75(1):1–7. PMID: 2045891.
4. Hayashi K, Kitaqawa N, Morikawa M, Hiu T, Morofuji Y, Suyama K, et al. MicroNester coil for neurointervention. J Neurosurg. 2009; 1. 110(1):40–43. PMID: 18821834.

5. Ihn YK, Sung JH, Byun JH. Antegrade recanalization of parent artery after internal trapping of ruptured vertebral artery dissecting aneurysm. J Korean Neurosurg Soc. 2012; 5. 51(5):301–304. PMID: 22792429.

6. Iihara K, Sakai N, Murao K, Sakai H, Higashi T, Kogure S, et al. Dissecting aneurysms of the vertebral artery : a management strategy. J Neurosurg. 2002; 8. 97(2):259–267. PMID: 12186451.
7. Jansen O, Dorfler A, Forsting M, Hartmann M, von Kummer R, Tronnier V, et al. Endovascular therapy of arteriovenous fistulae with electrolytically detachable coils. Neuroradiology. 1999; 12. 41(12):951–957. PMID: 10639676.

8. Jin SC, Kwon DH, Choi CG, Ahn JS, Kwun BD. Endovascular strategies for vertebrobasilar dissecting aneurysms. AJNR Am J Neuroradiol. 2009; 9. 30(8):1518–1523. PMID: 19474118.

9. Kai Y, Hamada JI, Morioka M, Todaka T, Mizuno T, Ushio Y. Endovascular coil trapping for ruptured vertebral artery dissecting aneurysms by using double microcatheters technique in the acute stage. Acta Neurochir (Wien). 003; 6. 145(6):447–451. discussion 451. PMID: 12836068.

10. Kim BM, Shin YS, Kim SH, Suh SH, Ihn YK, Kim DI, et al. Incidence and risk factors of recurrence after endovascular treatment of intracranial vertebrobasilar dissecting aneurysms. Stroke. 2011; 9. 42(9):2425–2430. PMID: 21778439.

11. Lemole GM Jr, Henn J, Javedan S, Deshmukh V, Spetzler RF. Cerebral revascularization performed using posterior inferior cerebellar artery-posterior inferior cerebellar artery bypass. Report of four cases and literature review. J Neurosurg. 2002; 7. 97(1):219–223. PMID: 12134919.
12. Lylyk P, Cohen JE, Ceratto R, Ferrario A, Miranda C. Combined endovascular treatment of dissecting vertebral artery aneurysms by using stents and coils. J Neurosurg. 2001; 3. 94(3):427–432. PMID: 11235947.

13. MacKay CI, Han PP, Albuquerque FC, McDougall CG. Recurrence of a vertebral artery dissecting pseudoaneurysm after successful stent-supported coil embolization: case report. Neurosurgery. 2003; 9. 53(3):754–759. discussion 760-1. PMID: 12943592.

14. Mizutani T, Aruga T, Kirino T, Miki Y, Saito I, Tsuchida T. Recurrent subarachnoid hemorrhage from untreated ruptured vertebrobasilar dissecting aneurysms. Neurosurgery. 1995; 5. 36(5):905–911. discussion 912-3. PMID: 7791980.

15. Rabinov JD, Hellinger FR, Morris PP, Ogilvy CS, Putman CM. Endovascular management of vertebrobasilar dissecting aneurysms. AJNR Am J Neuroradiol. 2003; 8. 24(7):1421–1428. PMID: 12917140.
16. Sugiu K, Tokunaga K, Watanabe K, Sasahara W, Ono S, Tamiya T, et al. Emergent endovascular treatment of ruptured vertebral artery dissecting aneurysms. Neuroradiology. 2005; 2. 47(2):158–164. PMID: 15703929.

17. Tsai CW, Lin TH, Ko CT, Chen MF, Lee YT. Transcatheter embolization of a coronary arteriovenous fistula with a complex, helical-fibered platinum coil. J Formos Med Assoc. 1996; 7. 95(7):558–561. PMID: 8840760.
18. Wakhloo AK, Lanzino G, Lieber BB, Hopkins LN. Stents for intracranial aneurysms: the beginning of a new endovascular era? Neurosurgery. 1998; 8. 43(2):377–379. PMID: 9696095.

Fig. 1
Computed tomography (CT) of the brain shows a more predominant subarachnoid hemorrhage in cisterns around the medullar and pons (A) than in the basal cistern (B).

Fig. 2
An 80-year-old male with a dissecting aneurysm. (A) CT angiography of the left vertebral artery shows irregular narrowing and a dilated portion of the dissecting aneurysm. (B) A CT source image shows a pseudoaneurysm and an intimal flap (arrow).
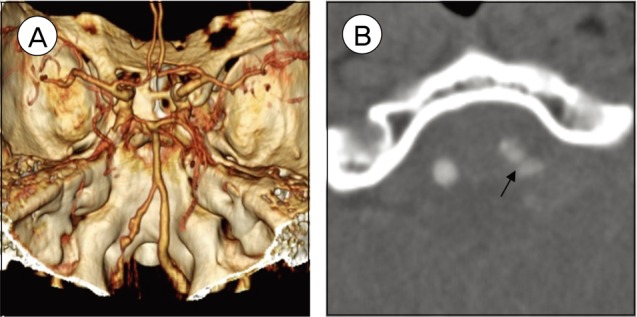
Fig. 3
Left vertebral angiogram shows a dissecting aneurysm. The left posterior inferior cerebellar artery is visualized at the distal portion of the aneurysm (arrow).
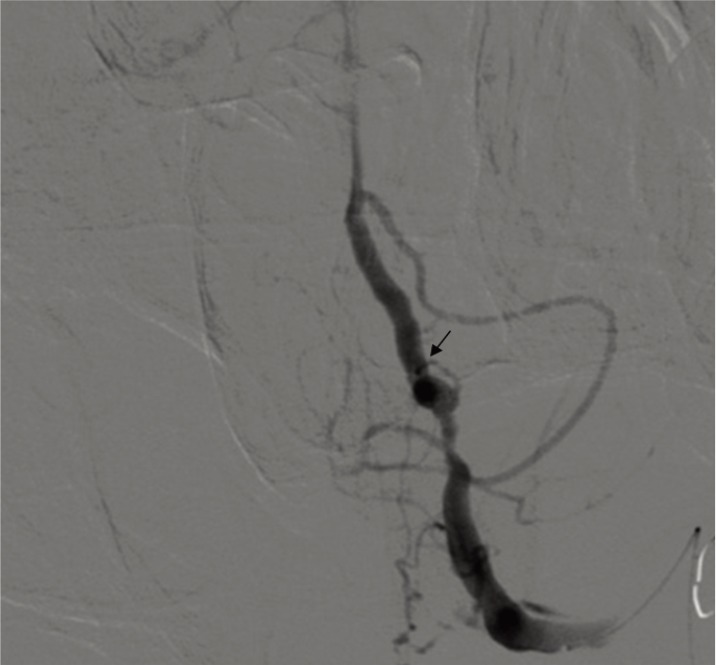
Fig. 4
(A) A microcatheter was navigated into the distal part of the delicate lesions and embolized using Guglielmi detachable coils (GDCs). (B) Left vertebral angiogram performed after GDCs embolization shows minimal filling of contrast in the trapped site using GDCs. (C) Two micro-tornado coils (3 mm × 2 mm) were deployed at the proximal portion of the trapped site (arrow). (D) The dissecting aneurysm and affected left vertebral artery are completely occluded, whereas several perforating arteries are preserved but the left posterior inferior cerebellar artery is sacrificed.
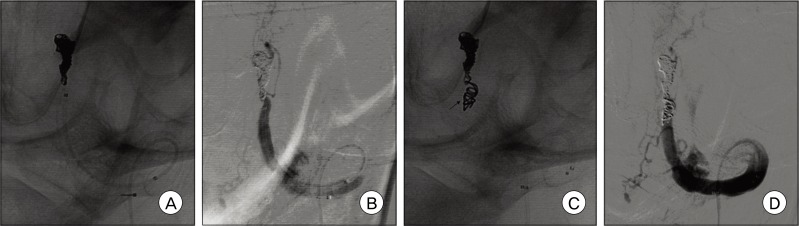
Fig. 5
(A and B) Postoperative magnetic resonance angiography shows complete occlusion of the left vertebral artery dissecting aneurysm with sufficient flow to the basilar artery via the right vertebral artery. (C) Follow-up magnetic resonance image shows embolic ischemic lesions in the left cerebellum.
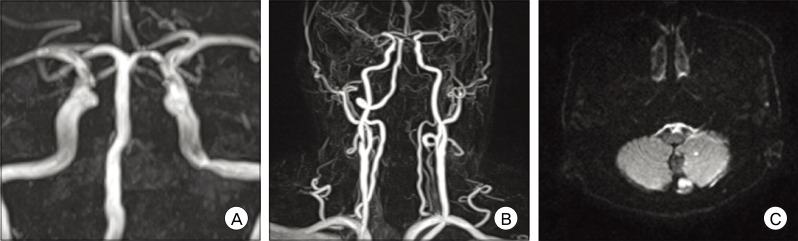
Fig. 6
(A) Photograph showing a platinum coil with synthetic fiber-coated micro-tornado coils (Tornado® Embolization Microcoil™, Cook incorporated, Bloomington, IN, proximal end diameter 3 mm × distal end diameter 2 mm, tornado-like cone shape). (B) A fiber-coated micro-nester coil (6 mm diameter). Synthetic fibers are coated transversely along the platinum coil.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download