Abstract
Objective
The purpose of this study is to investigate the results of treatment using stent-angioplasty for symptomatic middle cerebral arterial (MCA) stenosis and comparison of in-stent restenosis between drug-eluting stents (DES), bare metal coronary stents (BMS) and self-expanding stents (SES).
Materials and Methods
From Jan. 2007 to June. 2012, 34 patients (mean age ± standard deviation: 62.9 ± 13.6 years) with MCA stenosis were treated. Inclusion criteria were acute infarction or transient ischemic attacks (TIAs) and angiographically proven symptom related severe stenosis. Stents used for treatment were DES (n = 8), BMS (n = 13) and SES (n = 13). National Institutes of Health Stroke Scale (NIHSS) at admission was 2.5 ± 3.1 and mean stenosis rate was 79.0 ± 8.2%. Assessment of clinical and angiographic results was performed retrospectively.
Results
Among 34 patients, periprocedural complications occurred in four cases (11.8%), however, only two cases (6.0%) were symptomatic. All patients were followed clinically (mean follow-up period; 40.7 ± 17.7 months) and 31 were followed angiographically (91.2%. 13.4 ± 8.5 months). There was no occurrence of repeat stroke in all patients; however, mild TIAs related to restenosis occurred in three of 34 patients (8.8%). The mean NIHSS after stent-angioplasty was 1.7 ± 2.9 and 0.8 ± 1.1 at discharge. The modified Rankin score (mRS) at discharge was 0.5 ± 0.9 and 0.3 ± 0.8 at the last clinical follow-up. In-stent restenosis over 50% occurred in five of 31 angiographically followed cases (16.1%), however, all of these events occurred only in patients who were treated with BMS or SES. Restenosis rate was 0.0% in the DES group and 20.8% in the other group (p = 0.562); it did not differ between BMS and SES (2/11 18.2%, 3/13 23.1%, p = 1.000).
Intracranial atherosclerotic stenosis is responsible for approximately 5% to 10% of all strokes in mixed patient populations, and in the Asian population, this is even the most commonly found vascular lesion.10) Endovascular therapy using primary angioplasty or stenting has been used in treatment of medically refractory patients with high-grade intracranial atherosclerotic stenoses.5)22) However, endovascular management of intracranial stenosis is associated with a significant number of potential complications, including acute in-stent thrombosis, thromboembolism, vessel dissection, and even rupture. To date, according to some reports, these complications during angioplasty have varied widely, ranging from 0% to more than 30%.6)7)12)13) Therefore, current treatment decisions are usually based on results of case series for each center. Prior to introduction of self-expanding stents (SES) for intracranial stent-angioplasty, the only available stents for intracranial stenotic disease were coronary balloon mounted (balloon-expandable) stents. Since that time, many studies of intracranial stent-angioplasty have proceeded in earnest.3)8)11)14)18)27)30)38)
The main trunk of the middle cerebral artery (MCA) represents one of the most common sites of symptomatic intracranial atherosclerosis.4) However, stent-angioplasty for MCA lesions remains challenging because of vascular tortuosity, small vessel diameter, and concern for perforator injury. In addition, MCA lesions are known to be highly prone to restenosis after stent-angioplasty.32)
The authors assessed the treatment results, periprocedural complications, and angiographic results of stent-angioplasty for symptomatic MCA stenosis, and then attempted to compare the results of restenosis between drug-eluting stents (DES), bare metal coronary stents (BMS), and SES.
From January 2007 to June 2012, 35 patients (23 men, 12 women) underwent stent-angioplasty for MCA stenosis. Patients ranged from 34 to 84 years of age (mean ± standard deviation: 63.3 ± 13.6 years). We required that candidates for stent-angioplasty have acute or subacute symptomatic MCA territorial infarction (i.e. borderzone infarction) or repeated TIAs, and severe MCA stenotic rate over 70% related to symptoms confirmed with catheter angiography. Patients with asymptomatic stenosis or mild stenosis under 70% were excluded from endovascular treatment. Because of actual assessment for clinical and angiographic results of stent-angioplasty, an additional inclusion criterion for this study was successful deployment of a stent on the lesion. Therefore, 34 patients (23 male, 11 female, mean age: 62.9 ± 13.6 years) in whom stents were successfully deployed were included in this study. Stents used for treatment were DES (n = 8, 23.5%), BMS (n = 13, 38.2%), and SES (n = 13, 38.2%). The mean National Institutes of Health Stroke Scale (NIHSS) at admission was 2.5 ± 3.1, and mean stenosis rate was 79.0 ± 8.2%. Follow-up (FU) NIHSS was evaluated immediately after the procedure, and at discharge. Evaluation of modified Rankin Score (mRS) was performed at discharge and was last performed in the Out-Patient Department. Clinical status (including NIHSS and mRS) and angiographic results were assessed retrospectively. We then compared the results between DES, BMS, and SES.
Until Apr. 2010, DES or BMS was implanted for intracranial stenosis, including MCA stenosis, and, after that time, due to the availability of stents in domestic medical practice, most patients were treated with SES (Wingspan stent, Boston Scientific, Natick, MA).
All patients undergoing stent-angioplasty were given dual antiplatelet therapy for at least three days before the procedure, which consisted of aspirin (100 mg - 300 mg/d orally) and clopidogrel (75 mg/d orally), except for one patient, who underwent stent-angioplasty as an emergency treatment after a loading dose of dual antiplatelet medication (aspirin 300 mg and clopidogrel 375 mg). All endovascular procedures were performed under local anesthesia by a neurosurgeon and a neuroradiologist. A 6 or 7 French guiding catheter was inserted into the proximal internal cerebral artery (ICA) after a femoral puncture. A 0.014 inch (0.35 mm) wire was then introduced through the guiding catheter with or without use of a microcatheter. In cases of coronary balloon mounted stents, including DES and BMS, after the wire had passed through the stenotic lesion under the roadmap, the stent was delivered to the lesion along the wire and placed across the stenotic lesion by inflation of the balloon. The stent was deployed at the same time when the stenosis was dilated by inflation of the balloon. In cases of self-expanding stents (Wingspan stent), after the wire had passed through the lesion, a Gateway balloon was delivered to the lesion first, and inflated for pre-stent dilatation. A Wingspan stent was delivered to the lesion and placed across the lesion by unsheathing the stent-delivery catheter system after ballooning-dilatation. This procedure was performed under anticoagulation treatment with intravenous heparin, which was followed by nadroparin calcium (Fraxiparin; 2850 IU anti-Factor Xa, Sanofi Winthrop Industrie, Ambares, FR) dosed at 0.3 ml SC q 12 hours for at least one day following the procedure. Dual antiplatelet therapy was continued after the procedure and then switched to monotherapy after at least 12 weeks (aspirin 100 mg/d orally).
All digital subtraction angiograms were analyzed by a neurosurgeon. The angiographic view that best demonstrated the stenotic lesion was identified during pre-stenting angiography. This optimal view was used as the working view for stent placement and immediate post-stenting angiography. The angle of the chosen working view was recorded and reproduced during FU angiography (Fig. 1). The degree of stenosis was calculated as the percent stenosis from the catheter angiogram using the following formula:
Percent stenosis (%) = 100 × (1 - S/N), where S is the diameter of the residual lumen at the point of maximum stenosis and N is the width of the disease-free distal MCA (M1 segment) at the point where the walls were approximately parallel. Residual stenosis was quantified according to the vessel diameter achieved immediately after stent-angioplasty, and restenosis was calculated by comparing the lesion diameter of the follow up angiogram and that of the postoperative angiogram immediately after treatment. Restenosis was categorized into two groups: insignificant to mild restenosis (0-49%), and moderate to severe restenosis (50-100%).
Between-group comparisons were made according to the types of stents used. Age, sex, past history of medical disease (hypertension, diabetes mellitus, and hypercholesterolemia), angiographic data, including diameter of the disease-free distal MCA (reference diameter), length of stenosis, stenosis rate (preoperatively, postoperatively, and at FU angiography), and clinical status such as NIHSS and mRS were analyzed. We compared continuous variables using Student's t-tests and categorical variables using Pearson's Chi-Square tests. Comparisons were made according to confidence intervals of 95%. All statistical tests were two-sided and all analyses were performed using statistical software (SPSS for Windows, 15.0 standard version, IBM, NY). Values are expressed as mean ± standard deviation. A probability value less than 0.05 was considered statistically significant.
Of 35 patients, intracranial stents were successfully deployed at the first trial in 34 patients. In one patient, we attempted to perform stent-angioplasty using a Wingspan stent; stent delivery failed after successful pre-stent balloon angioplasty due to a severely tortuous ICA course. Therefore, we assessed the treatment results of stent-angioplasty with these 34 patients who were treated successfully. Overall results are shown in Table 1. NIHSS on the day after angioplasty showed improvement in most patients (2.5 ± 3.1 at admission vs. 1.7 ± 2.9 after the procedure, p = 0.014) and then continued to show improvement (0.8 ± 1.1, at discharge, p = 0.001) and so did mRS (0.5 ± 0.9 at discharge to 0.3 ± 0.8 at last FU, p = 0.003). Procedure-related complications occurred in four cases (11.8%); however, they were symptomatic in only two cases (5.8%). One was a case of MCA rupture after inflation of ballooning deployment of the coronary stent, and the other, a case of thromboembolism, appeared to be related to the procedure with a coronary stent not even detected angiographically during the procedure. Although the former was healed completely with the repetition of balloon inflation and deflation after stent deployment, a small amount of SAH and multiple scattered infarctions were developed in the MCA territory. And, in the latter, the aggravated hemiparesis showed improvement to a nearly previous state six months after the procedure. Two asymptomatic complications described above were proximal vessel (i.e. cervical segment of ICA) dissections caused by guiding catheters. In one case, it was healed spontaneously after the procedure, and the other was treated with another stent deployment without clinical sequela.
There were no cases of repeated infarction during the FU periods; however, mild TIAs occurred in three patients within one year after stent-angioplasty (7.2 ± 5.5 months). All of these patients' symptoms were mild but identical to preoperative ones, and proved to be related to severe in-stent restenosis over 70% on FU angiography. We attempted to retreat these patients with a drug-eluting balloon (DEB) in two of three patients, and with another stent in the other patient, because the stenotic lesion was located beside the center of the previously implanted stent. All of these patients have been in a symptom-free state for at least seven months after retreatment (40 and 14 months after retreatment with DEB, seven months with another stent, respectively).
The mean percent stenosis before the procedure was 79.0 ± 8.2%, and the reference diameter (vessel diameter just distal to lesion) was 2.5 ± 0.2 mm, and the length of the lesion was 6.7 ± 2.4 mm. The residual stenosis was 4.0 ± 9.2% immediately after stent deployment. FU angiograms were obtained in 31 of 34 patients (91.2%) at a mean of 13.4 ± 13.4 months after the procedure. There were no differences in the group characteristics between whole patients who had undergone stent-angioplasty and the patients who have been conducted angiographic FU either (Table 1).
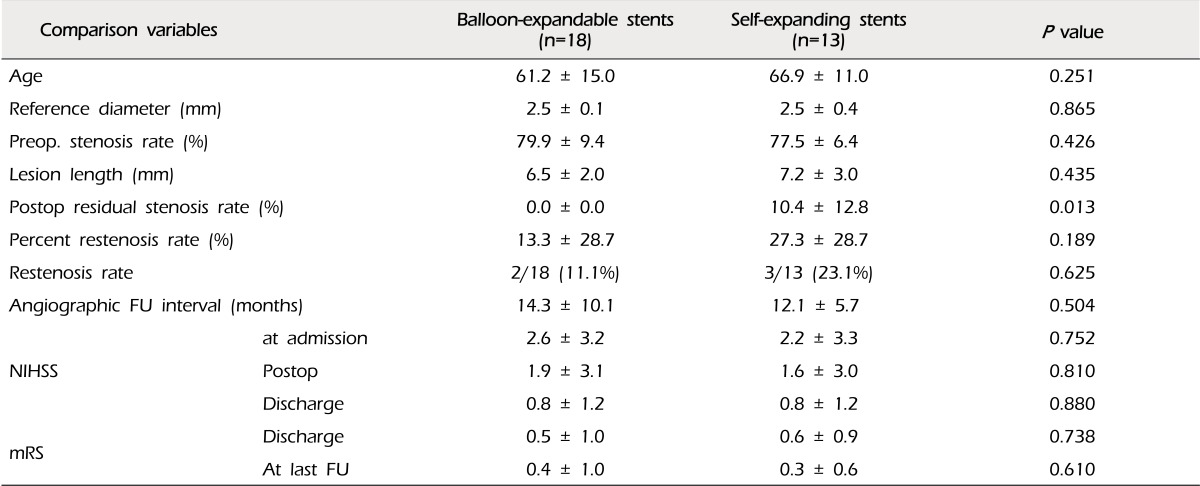
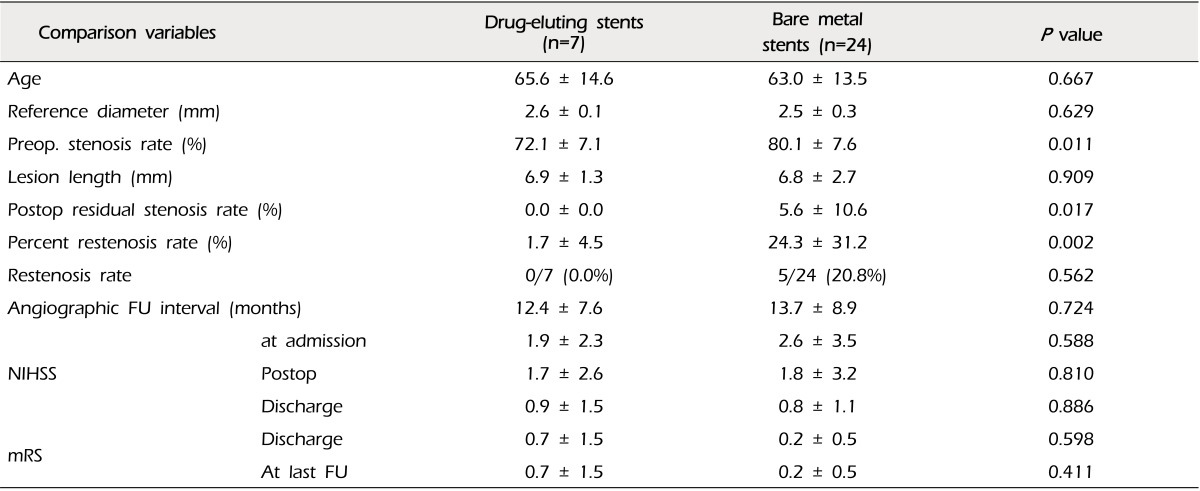
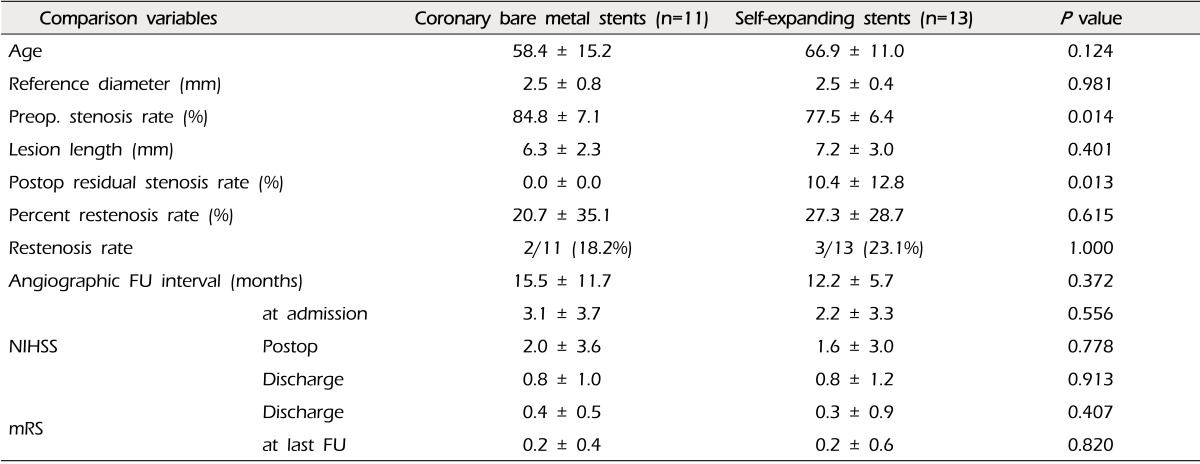
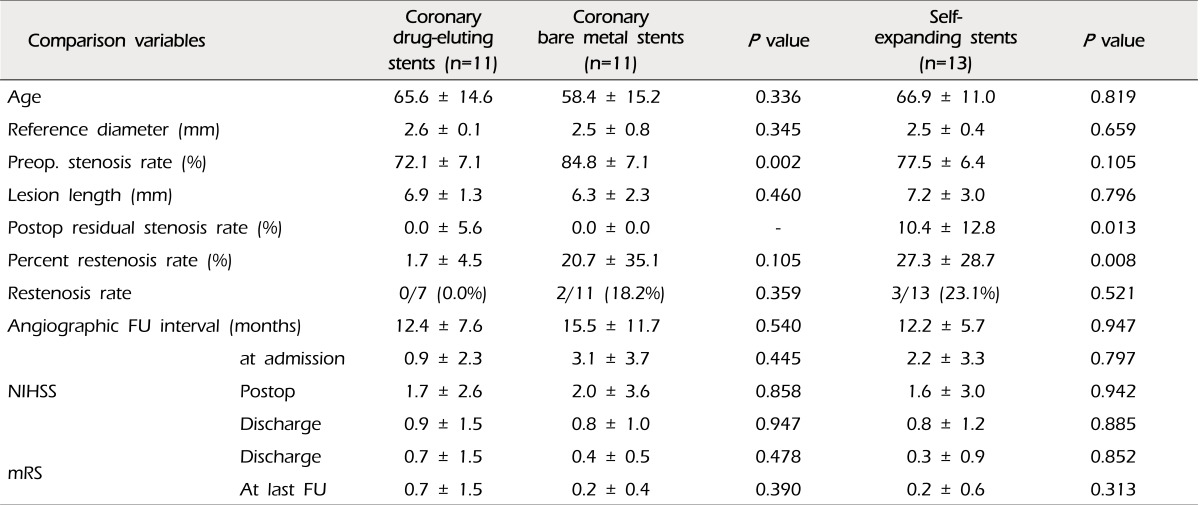
The mean percent of restenosis was 19.2 ± 29.1% on FU angiograms. Each of the documented restenosis occurred as "in-stent" restenosis, as a sequela of thickening of the intima into the stent strut, not as a result of stent recoil or stent kinking. On the other hand, the overall rate of moderate-to-severe restenosis (≥ 50%) was 16.1% (five of 31 cases). Two cases were patients treated with coronary balloon-expandable stents, and the other three patients were treated with the SES (2/18, 11.1% vs. 4/13, 23.1%, p = 0.625). Therefore, we compared the results between the groups of patients who were treated with coronary balloon-expandable stents and SES (Table 2). In this comparison, we found that only one variable differed i.e. postoperative residual stenosis rate, even though the difference of restenosis rate itself did not reach statistical significance. There were no cases of restenosis in patients treated with DES. Thus, we compared patients treated with DES and the others (Table 3). Some variables differed between these two groups. Postoperative residual stenosis rate and percent restenosis rate differed significantly. We then compared the BMS and SES, because both stents have the same characteristics in terms of the bare metal stent, but not the same design, such as balloon-expandable stents vs. self-expanding stents, which should be deployed after suboptimal balloon angioplasty (Table 4). In this comparison, the only significant difference was postoperative residual stenosis. However, in comparison of this between DES vs. BMS, it was nearly the same, but the percent restenosis rate was different, although it did not reach statistical significance (DES vs. BMS, 1.7 ± 4.5 vs. 20.7 ± 35.1, p = 0.105) (Table 5). Meanwhile, in cases of SES, both were significantly different from DES (Postoperative residual stenosis: 10.4 ± 12.8, p = 0.013, Percent restenosis rate: 27.3 ± 28.7%, p = 0.008) (Table 5). No association was observed between development of restenosis and past history of medical disease, including hypertension (p = 0.394), diabetes mellitus (p = 1.000), and hypercholesterolemia (p = 1.000).
The success rate of stent-angioplasty was 97.1% in this study, and the procedural complication rate was 5.7% in the case of symptomatic patients. No infarction or hemorrhage was observed over one-year follow-up periods. The reported success rate and procedural risk of intracranial angioplasty varied according to the reports.9)36) Yoon et al.36) reported risk of disabling stroke or death of 6% (two of 32 patients), and mortality rate of 3% (one of 32 patients). Marks et al.20) reported a rate of periprocedural death and stroke of 8.3%, and, in a Taiwan study, Jiang et al.15) reported a complication rate up to 11.8%. In a systemic review in 2009, the success rate of stent angioplasty for intracranial arterial stenosis was reported from 71.4% to 100%, and the periprocedural minor or major stroke and death rates ranged from 0% to 50% with a median of 7.7%.13) Meanwhile, Zhang et al.37) reported feasible results of stent-angioplasty for MCA stenosis. Although the size of the study was relatively smaller than that of the previous reports, in comparison, the author's result appears to show an equivalent or slightly better clinical outcome.
As for the restenosis, in the Stenting of Symptomatic Atherosclerotic Lesions in the Vertebral or Intracranial Arteries (SSYLVIA) Study, the rate of successful stent placement was 95%, however, restenosis rate of ≥ 50% at six months was 32.4%.31) Recently, the incidence of in-stent restenosis and retreatment of this condition were intensively investigated from data of the US Wingspan Registry, and the rate of restenosis was reported as 29.8%, and an additional 4.5% of stent thromboses occurred during an average follow up period of 5.9 months.19) The rate of recurrent stroke in the subgroup with in-stent restenosis was 6.8% and the rate of TIA was 13.8%.19) In this study, overall restenosis over 50% was 16.1% (five of 31 cases) and only 9.7% (3/31) for symptomatic cases at a mean angiographic follow up period of 13.4 months. However, due to the diverse composition of the kinds of stents, these figures should not be taken at face value. All cases of restenosis were bare metal stent, including coronary BMS and SES, and in case of SES (Wingspan stent), restenosis rate was 23.1%. This result is not much better than that of previous reports. However, on the contrary, restenosis did not occur in cases with DES, although the number of cases was very small (Fig. 2). DES has been known to be effective for reducing the risk of in-stent restenosis due to its controlled local release of antiproliferative agents, as compared with BMS in coronary stenting.23)28)29) Off-label use of drug-eluting coronary stents for treatment of intracranial atherosclerotic lesions has recently been reported.1)6)27) Park et al.24) reported promising results on drug-eluting stents for intracranial stenosis. The DES appears to be a good option for prevention of in-stent restenosis, however, technical difficulty due to stent stiffness remains a serious problem in generalizing and drawing definitive conclusions regarding the safety and effectiveness for intracranial lesions, especially distal ICA or MCA lesions, although restenosis itself was solved in part with these stents.16)33)
Findings of this study revealed a tendency of a similar rate of restenosis in cases of coronary BMS and SES (18.2% at BMS, 23.1% at SES, p = 1.000) (Table 4). The only meaningful difference was immediate postoperative residual stenosis rate. Percentage of residual stenosis in cases of balloon expandable stents such as DES and BMS was nearly zero, however, in case of SES, it was over 10%. This might be due to the prestent balloon angioplasty without simultaneous stenting of the Gateway Wingspan system. Technically, it places restrictions on sufficient dilatation of the lesions because of concern regarding serious arterial injury, such as dissection and even rupture. However, because of the similar results of the coronary BMS and SES, it might not be a risk factor. On the other hand, Shin et al.26) pointed out the speed of inflation as a risk factor for in-stent restenosis in Wingspan stenting. Although this explanation could have important implications for Wingspan stenting, it may not be applicable in the same way in cases of stenting with balloon-expandable coronary stents, because their use required significantly less stenting time (approximately 20 to 30 seconds in the author's series). Further studies should be required in order to clarify the association between in-stent restenosis and relevant risk factors, including technical aspects mentioned above, and other medical history.
In cases of restenosis, we attempted to administer retreatment only in symptomatic patients (three of five cases). In one case, retreatment was administered using another Wingspan system, because the stenotic lesion was located beside the center of the previously implanted stent. We treated the other two patients with the DEB (Fig. 3). All of these patients have been in a symptom-free state for at least seven months since retreatment (40 and 14 months after retreatment with a drug-eluting balloon, seven months with another stent, respectively). Several reported options, such as balloon angioplasty, another stent-angioplasty, etc, can be adopted for retreatment of restenosis. Currently, balloon angioplasty has been adopted in the majority of cases.12) Recently, the combination of mechanical balloon dilatation and local drug application was confirmed to be very efficient for treatment of coronary in-stent restenosis, and Vajda et al.34) reported successful treatment results of a DEB for the patients with in-stent restenosis after stent-angioplasty for intracranial stenotic diseases. In addition, they introduced another combination of DEB followed by the SES, instead of a current bare balloon with a bare stent, such as a Gateway balloon and Wingspan stent.35) Although conduct of further studies is needed, the DEB appears to be a promising alternative option for in-stent restenosis.
In this study, stent-angioplasty appeared to be safe and effective for MCA stenotic disease, however, a limitation of the current study was that the number of patients included was relatively small. It should prompt further studies on a larger scale.
The rate of in-stent restenosis was 16.1% as a whole, however, DES showed better results for prevention of restenosis, compared with BMS or SES. In this study, although the postoperative residual stenosis was one of the most noticeable differences between coronary balloon-expandable stents and SES, it appears to have little direct relationship with in-stent restenosis.
References
1. Abou-Chebl A, Bashir Q, Yadav JS. Drug-eluting stents for the treatment of intracranial atherosclerosis: initial experience and midterm angiographic follow-up. Stroke. 2005; 12. 36(12):e165–e168. PMID: 16282539.

2. Albuquerque FC, Levy EI, Turk AS, Niemann DB, Aagaard-Kienitz B, Pride GL Jr, et al. Angiographic patterns of Wingspan in-stent restenosis. Neurosurgery. 2008; 7. 63(1):23–27. PMID: 18728565.

3. Al Hasan M, Murugan R. Stenting versus aggressive medical therapy for intracranial arterial stenosis: more harm than good. Crit Care. 2012; 5. 16(3):310. PMID: 22574950.

4. Arenillas JF, Molina CA, Montaner J, Abilleira S, González-Sánchez MA, Alvarez-Sabín J. Progression and clinical recurrence of symptomatic middle cerebral artery stenosis: a long-term follow-up transcranial Doppler ultrasound study. Stroke. 2001; 12. 32(12):2898–2904. PMID: 11739993.
5. Bose A, Hartmann M, Henkes H, Liu HM, Teng MM, Szikora I, et al. A novel, self-expanding, nitinol stent in medically refractory intracranial atherosclerotic stenoses: the Wingspan study. Stroke. 2007; 5. 38(5):1531–1537. PMID: 17395864.
6. Boulos AS, Agner C, Deshaies EM. Preliminary evidence supporting the safety of drug-eluting stents in neurovascular disease. Neurol Res. 2005; 27(Suppl 1):S95–S102. PMID: 16197833.

7. Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011; 9. 365(11):993–1003. PMID: 21899409.
8. Derdeyn CP, Fiorella D, Lynn MJ, Barnwell SL, Zaidat OO, Meyers PM, et al. Impact of operator and site experience on outcomes after angioplasty and stenting in the SAMMPRIS trial. J Neurointerv Surg. 2012; 9. 12.

9. De Rochemont Rdu M, Turowski B, Buchkremer M, Sitzer M, Zanella FE, Berkefeld J. Recurrent symptomatic high-grade intracranial stenoses: safety and efficacy of undersized stents-initial experience. Radiology. 2004; 4. 231(1):45–49. PMID: 15068940.
10. De Silva DA, Woon FP, Lee MP, Chen CP, Chang HM, Wong MC. South Asian patients with ischemic stroke: intracranial large arteries are the predominant site of disease. Stroke. 2007; 9. 38(9):2592–2594. PMID: 17656660.
11. Fiorella D, Levy EI, Turk AS, Albuquerque FC, Niemann DB, Aagaard-Kienitz B, et al. US multicenter experience with the wingspan stent system for the treatment of intracranial atheromatous disease: periprocedural results. Stroke. 2007; 3. 38(3):881–887. PMID: 17290030.

12. Fiorella D, Levy EI, Turk AS, Albuquerque FC, Pride GL Jr, Woo HH, et al. Target lesion revascularization after wingspan: assessment of safety and durability. Stroke. 2009; 1. 40(1):106–110. PMID: 18927447.
13. Gröschel K, Schnaudigel S, Pilgram SM, Wasser K, Kastrup A. A systematic review on outcome after stenting for intracranial atherosclerosis. Stroke. 2009; 5. 40(5):e340–e347. PMID: 19182081.

14. Henkes H, Miloslavski E, Lowens S, Reinartz J, Liebig T, Kühne D. Treatment of intracranial atherosclerotic stenoses with balloon dilatation and self-expanding stent deployment (Wingspan). Neuroradiology. 2005; 3. 47(3):222–228. PMID: 15912418.

15. Jiang WJ, Du B, Leung TW, Xu XT, Jin M, Dong KH. Symptomatic Intracranial Stenosis: cerebrovascular complications from elective stent placement. Radiology. 2007; 4. 243(1):188–197. PMID: 17392253.

16. Kim JS, You SH, Kim SR, Kim SD, Kim YW, Park IK, et al. Usefulness of drug-eluting stents in angioplasty and stenting of the vertebral artery origin: comparison with bare stents. Korean J Cerebrovasc Surg. 2005; 7(2):135–142.
17. Lee J, Kwon S, Lee JH, Suh DC, Kim JS. Percutaneous transluminal angioplasty for symptomatic middle cerebral artery stenosis: long-term follow-up. Cerebrovasc Dis. 2003; 15(1-2):90–97. PMID: 12499717.

18. Lee JH, Yun JK, Kim DW, Kang SD. Clinical and angiographic outcomes of Wingspan stent placement for treatment of symptomatic intracranial stenosis: single center experience with 19 cases. J Cerebrovasc Endovasc Neurosurg. 2012; 9. 14(3):157–163. PMID: 23210041.

19. Levy EI, Turk AS, Albuquerque FC, Niemann DB, Aagard-Kienitz B, Pride L, et al. Wingspan in-stent restenosis and thrombosis: incidence, clinical presentation, and management. Neurosurgery. 2007; 9. 61(3):644–650. discussion 650-1. PMID: 17881980.
20. Marks MP, Marcellus ML, Do HM, Schraedley-Desmond PK, Steinberg GK, Tong DC, et al. Intracranial angioplasty without stenting for symptomatic atherosclerotic stenosis: long-term follow-up. AJNR Am J Neuroradiol. 2005; 3. 26(3):525–530. PMID: 15760860.
21. Marks MP, Marcellus M, Norbash AM, Steinberg GK, Tong D, Albers GW. Outcome of angioplasty for atherosclerotic intracranial stenosis. Stroke. 1999; 5. 30(5):1065–1069. PMID: 10229745.

22. Marks MP, Wojak JC, Al-Ali F, Jayaraman M, Marcellus ML, Connors JJ, et al. Angioplasty for symptomatic intracranial stenosis: clinical outcome. Stroke. 2006; 4. 37(4):1016–1020. PMID: 16497979.
23. Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002; 6. 346(23):1773–1780. PMID: 12050336.

24. Park S, Lee DG, Chung WJ, Lee DH, Suh DC. Long-term outcomes of drug-eluting stents in symptomatic intracranial stenosis. Neurointervention. 2013; 2. 8(1):9–14. PMID: 23515851.

25. Scheller B, Hehrlein C, Bocksch W, Rutsch W, Haghi D, Dietz U, et al. Two year follow-up after treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. Clin Res Cardiol. 2008; 10. 97(10):773–781. PMID: 18536865.

26. Shin YS, Kim BM, Suh SH, Jeon P, Kim DJ, Kim DI, et al. Wingspan stenting for intracranial atherosclerotic stenosis: clinical outcomes and risk factors for in-stent restenosis. Neurosurgery. 2013; 4. 72(4):596–604. discussion 604. PMID: 23277374.
27. Steinfort B, Ng PP, Faulder K, Harrington T, Grinnell V, Sorby W, et al. Midterm outcomes of paclitaxel-eluting stents for the treatment of intracranial posterior circulation stenoses. J Neurosurg. 2007; 2. 106(2):222–225. PMID: 17410703.

28. Stettler C, Wandel S, Allemann S, Kastrati A, Morice MC, Schömig A, et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007; 9. 370(9591):937–948. PMID: 17869634.

29. Stone GW, Ellis SG, Cox DA, Hermiller J, OShaughnessy C, Mann JT, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004; 1. 350(3):221–231. PMID: 14724301.

30. Tarlov N, Jahan R, Saver JL, Sayre JW, Ali LK, Kim D, et al. Treatment of high risk symptomatic intracranial atherosclerosis with balloon mounted coronary stents and Wingspan stents: single center experience over a 10 year period. J Neurointerv Surg. 2012; 1. 4(1):34–39. PMID: 21990460.

31. The SSYLVIA Study Investigators. Stenting of symptomatic atherosclerotic lesions in the vertebral or intracranial arteries (SSYLVIA): study results. Stroke. 2004; 6. 35(6):1388–1392. PMID: 15105508.
32. Turk AS, Levy EI, Albuquerque FC, Pride GL Jr, Woo H, Welch BG, et al. Influence of patient age and stenosis location on wingspan in-stent restenosis. AJNR Am J Neuroradiol. 2008; 1. 29(1):23–27. PMID: 17989366.

33. Vajda Z, Aguilar M, Göhringer T, Horváth-Rizea D, Bäzner H, Henkes H. Treatment of intracranial athero sclerotic disease with a balloon-expandable paclitaxel eluting stent: procedural safety, efficacy and mid-term patency. Clin Neuroradiol. 2012; 9. 22(3):227–233. PMID: 22252289.
34. Vajda Z, Güthe T, Perez MA, Heuschmid A, Schmid E, Bäzner H, et al. Neurovascular in-stent stenoses: treatment with conventional and drug-eluting balloons. AJNR Am J Neuroradiol. 2011; Nov-Dec. 32(10):1942–1947. PMID: 21885715.

35. Vajda Z, Güthe T, Perez MA, Kurre W, Schmid E, Bäzner H, et al. Prevention of intracranial in-stent restenoses: predilatation with a drug eluting balloon, followed by the deployment of a self-expanding stent. Cardiovasc Intervent Radiol. 2013; 4. 36(2):346–352. PMID: 22869043.

36. Yoon W, Seo JJ, Cho KH, Kim MK, Kim BC, Park MS, et al. Symptomatic middle cerebral artery stenosis treated with intracranial angioplasty: experience in 32 patients. Radiology. 2005; 11. 237(2):620–626. PMID: 16192322.

37. Zhang L, Huang Q, Zhang Y, Deng B, Liu J, Hong B, et al. A single-center study of Wingspan stents for symptomatic atherosclerotic stenosis of the middle cerebral artery. J Clin Neurosci. 2013; 3. 20(3):362–366. PMID: 23228656.

38. Zhou Y, Yang QW, Xiong HY. Angioplasty with stenting for intracranial atherosclerosis: a systematic review. J Int Med Res. 2012; 2. 40(1):18–27. PMID: 22429342.

Fig. 1
Catheter angiograms of a 66-year-old male patient who presented with repeated transient ischemic attacks of left arm weakness. (A) Preoperative right internal cerebral artery (ICA) angiogram demonstrates severe stenosis at the right middle cerebral artery (MCA) and flow compromise of the MCA territory. (B) Right MCA was fully recanalized after Wingspan stent (Boston Scientific, Natick, MA, USA) deployment. (C) Follow-up angiogram three months after stent-angioplasty. M1 segment in which stent was implanted is more remodeled than immediately after stent-angioplasty. (D) Follow-up angiogram 13 months after stent-angioplasty. No change is seen when compared with the three month follow-up angiogram.
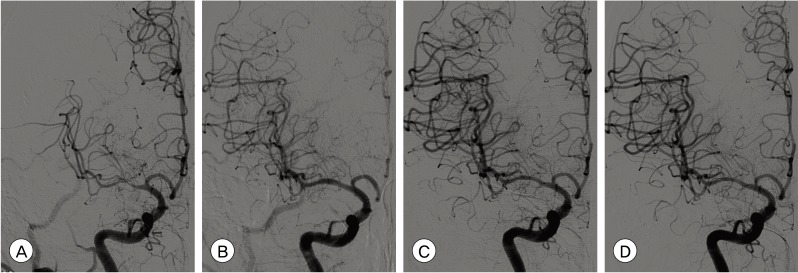
Fig. 2
Catheter angiograms of a 68-year-old female patient who presented with MCA borderzone infarct. (A) Preoperative left ICA angiogram demonstrates severe stenosis at M1 segment and definite flow compromise caused by the stenosis. (B) Postoperative angiogram after stent-angioplasty with a drug-eluting coronary stent (Endeavor, Medtronic Vascular, Santa Rosa, CA) shows no residual stenosis and fully recovered MCA flow. (C) One year follow-up angiogram shows no restenosis compared with immediate postoperative angiogram.
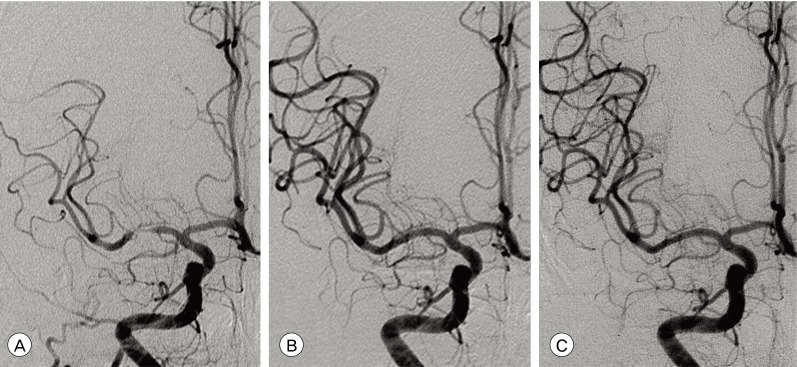
Fig. 3
Catheter angiograms of a 47-year-old male patient who presented with right MCA borderzone infarct. (A) Preoperative right ICA angiogram demonstrates MCA occlusion. (B) Postoperative angiogram after stent-angioplasty with a bare metal coronary stent (Coroflex, B. Braun, DE) shows no residual stenosis and fully recovered MCA flow. (C) Three month follow-up angiogram shows severe in-stent restenosis up to 80% with mild flow compromise. (D) Follow-up angiogram immediately after retreatment (balloon angioplasty) with a drug-eluting balloon (SeQuent® Please, B.Braun, DE) shows fully recovered MCA flow compromise, but insignificant residual stenosis remained. (E) One year follow-up angiogram after retreatment demonstrates a fully dilated M1 segment without any residual stenosis or restenosis.
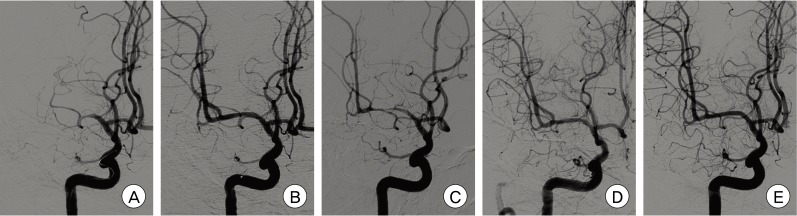
Table 1
Comparisons of all patients who had undergone stent-angioplasty and the patients who have been conducted angiographic follow-up either
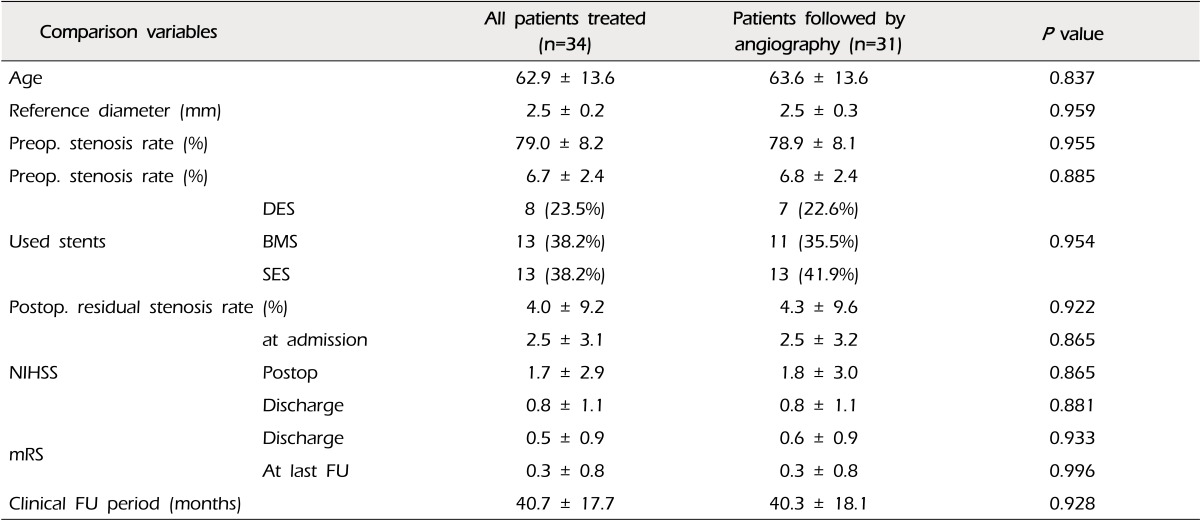
Table 2
Comparisons of patients treated with coronary balloon-expandable stents (including drug-eluting stents and bare metal stents) vs. self-expanding stents.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download