Abstract
We report a rare case of an idiopathic pseudoaneurysm causing intraventricular hemorrhage (IVH). A 28-year-old man presented with sudden onset of severe headache. He underwent external ventricular drainage for an isolated IVH in the right lateral ventricle. Digital subtraction angiography (DSA) revealed that the aneurysm (7.5×4.5 mm) arose from the distal part of the medial lenticulostriate artery. Following removal of the external ventricular drainage catheter, the aneurysm decreased in size (4.0×2.3 mm). However, follow-up DSA revealed a slightly enlarged aneurysm (4.2×3.2 mm) with morphologic change. The aneurysm was clipped via the interhemispheric transcallosal approach, but postoperative DSA revealed a residual aneurysm sac beside the clips. Given the risk of rebleeding, a second operation was planned for complete resection of the aneurysm. After revised craniotomy and careful dissection of the caudate nucleus, the aneurysm sac was completely resected. Histopathological examination revealed that the aneurysm was a pseudoaneurysm. The patient recovered without any neurological sequel and was discharged.
To the best of our knowledge, this is the first reported case of an idiopathic lenticulostriate artery pseudoaneurysm protruding into the right lateral ventricle and causing an IVH that was successfully treated with microsurgical resection.
Intraventricular aneurysms (IVA) associated with intraventricular hemorrhage (IVH) are rare. IVAs typically occur secondary to Moyamoya disease (MMD), head trauma, intracranial arteriovenous malformations (AVM), and infection. A recent review by Yuan et al. estimated the prevalence of these conditions to be: MMD (50.9%), hypertension (15.1%), AVM (5.7%), and tuberculous infection (1.9%). Idiopathic IVAs accounted for 26.4% of all cases.13) Most reports of idiopathic IVAs describe true aneurysms, while only 1 report described the presence of a superior cerebellar artery pseudoaneurysm protruding into the fourth ventricle.7)
To the best of our knowledge, this is the first reported case of an idiopathic lenticulostriate artery (LSA) pseudoaneurysm protruding into the right lateral ventricle and causing an IVH that was successfully treated with microsurgical resection.
A 28-year-old man presented with sudden onset of severe headache without any definite history of trauma. He was previously diagnosed with bipolar disorder and had been taking lithium for 3 years. Computed tomography (CT) scans revealed isolated IVH in the right lateral ventricle. An enhanced small round lesion (4.5 mm in diameter) was found on the medial surface of the right caudate nucleus by contrast CT (Fig. 1). The patient underwent external ventricular drainage (EVD) for the IVH. The following day, digital subtraction angiography (DSA) was performed and revealed an aneurysm arising from the distal part of the medial LSA (Fig 2A). Using 3-dimensional reconstruction images, the aneurysm was measured to be 7.5×4.5 mm. There was no evidence of MMD or AVM. The patient recovered without any newly developed neurological deficits and a follow-up CT scan showed complete resolution of the IVH.
Following removal of the EVD catheter, the size of the aneurysm slightly decreased (4.0×2.3 mm). A short-term follow-up of the aneurysm was recommended, to which the patient agreed. However, DSA performed a month later revealed a slightly enlarged aneurysm (4.2×3.2 mm). Moreover, the shape of aneurysm was changed. Considering the risk of rebleeding, we recommended surgical treatment. Endovascular obliteration of the aneurysm was deemed impossible due to the small caliber of the LSA. Instead, the aneurysm was clipped via the interhemispheric transcallosal approach with assistance of a neuro-navigation system. The aneurysm was found in the right lateral ventricle, attached to the caudate nucleus (Fig. 3A). To prevent injury to the caudate nucleus, we decided to clip the neck of the aneurysmal sac rather than undertake a complete resection. This was particularly important since the patient did not present any neurological deficits prior to surgery. A protruding pulsating sac partially enveloped by a hematoma was observed, and 2 aneurysm clips were successfully applied to the most proximal part of the sac (Fig. 3B). The patient recovered well.
DSA performed 4 days later revealed a considerable residual aneurysm sac beside the clips (Fig. 2B). Given the risk of rebleeding, a second operation was planned for complete resection of the aneurysm. After revised craniotomy and careful dissection of the caudate nucleus, the aneurysm sac and clips were completely removed (Fig. 3C). DSA after revised craniotomy showed no evidence of aneurysm (Fig. 2C). Short- and long-term memory tests were performed, and memory impairment was not observed. Fortunately, the patient recovered without any neurological sequelae.
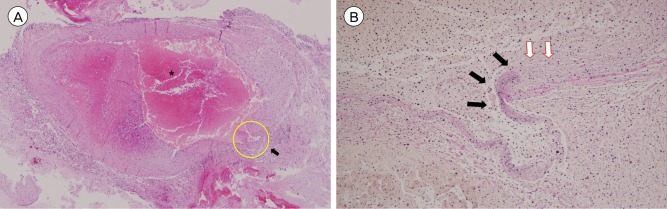
Histopathological examination was conducted on the resected aneurysm. Hematoxylin-eosin staining revealed partially organized thrombi surrounded by a collagen wall. Examination of the vascular wall structure by elastin staining failed to show an elastin layer around the hematoma. The aneurysm was subsequently diagnosed as a pseudoaneurysm (Fig. 4). Since there was no evidence or history of recent head trauma, this case was regarded as idiopathic LSA pseudoaneurysm causing IVH.
In our patient, enhanced CT angiography revealed an enhanced nodular lesion without evidence of vascular connections. Additional magnetic resonance imaging (MRI) showed peripheral T1-weighted hyperintensity, with a laminated pattern of mixed T1 signal and T2 hypointensity related to blood flow. These findings are similar to a previous neuroimaging study of a sphenoid sinus internal carotid artery pseudoaneurysm.8) However, in our case, the extent and origin of the aneurysm could not be identified with MRI. Instead, the most useful information for diagnosis and treatment was obtained by DSA. Initial DSA revealed the aneurysm (7.5×4.5 mm) arose from the distal part of the medial LSA.
The treatment goal for IVAs is to prevent rebleeding. A literature review showed that 14 out of 15 patients presenting with idiopathic IVAs underwent surgical or endovascular treatment, and their prognoses were relatively better than the patient who received conservative treatment: this patient died due to rebleeding.5) Moreover, in cases of IVA associated with MMD, 2 patients died during conservative management.4) These findings support active treatment of patients with IVH presenting with IVAs, but in this case, the patient did not present any symptoms after EVD care. Short-term follow up was decided after careful consideration because surgical or endovascular treatment could lead adverse effect. However, treatment of IVA became crucial because follow-up DSA performed a month later highlighted changes in the aneurysm's shape and size.
Endovascular treatment can be considered because endovascular intervention seems to improve the prognosis for traumatic and mycotic intracranial pseudoaneurysms.1)2) Among idiopathic IVA cases, 4 cases who received endovascular treatment showed good outcome.3)9)12)13) Endovascular embolization with embolic materials like glue and Onyx are usually used.6) However, there are some disadvantages to this technique. Since most IVAs arise from small distal branches as in this case, it is usually difficult to navigate a microcatheter accurately. Moreover, accidental reflux of the embolic source may result in embolization of an unintended vessel.
We decided to apply clips at the neck of aneurysm to avoid tissue damage of caudate nucleus during exploration of parent artery, but IVA remained. Two previous reports of successful clipping relate to the parent arteries being visibly located at the tela choroidea.10)11) Furthermore, pseudoaneurysms usually consist of a ruptured vessel wall and an extravascular hematoma covered in thin connective tissue. Given the anatomic features of these aneurysms, placement of clips is challenging.6) Except for these special cases, trapping of the aneurysm is essential for complete obliteration of the aneurysm from the parent circulation.
References
1. Chapot R, Houdart E, Saint-Maurice JP, Aymard A, Mounayer C, Lot G, et al. Endovascular treatment of cerebral mycotic aneurysms. Radiology. 2002; 2. 222(2):389–396. PMID: 11818604.

2. Cohen JE, Gomori JM, Segal R, Spivak A, Margolin E, Sviri G, et al. Results of endovascular treatment of traumatic intracranial aneurysms. Neurosurgery. 2008; 9. 63(3):476–485. discussion 485-6. PMID: 18812959.

3. Inci S, Arat A, Ozgen T. Distal anterior choroidal artery aneurysms. Surg Neurol. 2007; 1. 67(1):46–52. discussion 52. PMID: 17210297.

4. Konishi Y, Kadowaki C, Hara M, Takeuchi K. Aneurysms associated with moyamoya disease. Neurosurgery. 1985; 4. 16(4):484–491. PMID: 3990927.

5. Madhugiri VS, Gundamaneni SK, Yadav AK, Sasidharan GM, Roopesh KV. Idiopathic intraventricular aneurysm presenting with intraventricular hemorrhage: Case report and review of the literature. Pediatr Neurosurg. 2012; 48(3):174–180. PMID: 23406825.

6. Medel R, Crowley RW, Hamilton DK, Dumont AS. Endovascular obliteration of an intracranial pseudoaneur ysm: the utility of Onyx. J Neurosurg Pediatr. 2009; 11. 4(5):445–448. PMID: 19877777.
7. Miyake H, Ohta T, Kajimoto Y, Ogawa R, Deguchi J. Intraventricular aneurysms - Three case reports. Neurol Med Chir (Tokyo). 2000; 1. 40(1):55–60. PMID: 10721256.
8. Saket RR, Hetts SW, Tatum JK, Glastonbury CM. CT and MRI findings of sphenoid sinus internal carotid artery pseudoaneurysm: An important and challenging differential diagnosis for a skull base mass. Clin Radiol. 2012; 8. 67(8):815–820. PMID: 22336670.

9. Sanli AM, Cekirge S, Sekerci Z. Aneurysm of the distal anterior cerebral artery radiologically mimicking a ventricular mass. J Neurosurg. 2011; 4. 114(4):1061–1064. PMID: 20635851.
10. Ungersbock K, Perneczky A. Intraventricular aneurysm of the medial posterior choroid artery clipped via the contralateral transcallosal approach. Acta Neurochir (Wien). 1986; 82(1-2):24–27. PMID: 3751701.
11. Urbach H, Meyer B, Cedzich C, Solymosi L. Posterior inferior cerebellar artery aneurysm in the fourth ventricle. Neuroradiology. 1995; 5. 37(4):267–269. PMID: 7666957.

12. Weigele JB, Chaloupka JC, Lesley WS, Mangla S, Hitchon PW, VanGilder JC, et al. Peripheral aneurysms of the lateral posterior choroidal artery: clinical presentation and endovascular treatment: Report of two cases. Neurosurgery. 2002; 2. 50(2):392–395. discussion 395-6. PMID: 11844276.

13. Yuan Z, Woha Z, Weiming X. Intraventricular aneurysms: case reports and review of the literature. Clin Neurol Neurosurg. 2013; 1. 115(1):57–64. PMID: 22633465.

Fig. 1
Initial enhanced computed tomography images. Pure intraventricular hemorrhage is observed in the right lateral ventricle. Small nodular enhancing lesion is located beside head of caudate nucleus (Arrow: enhanced aneurysm, H: hematoma).
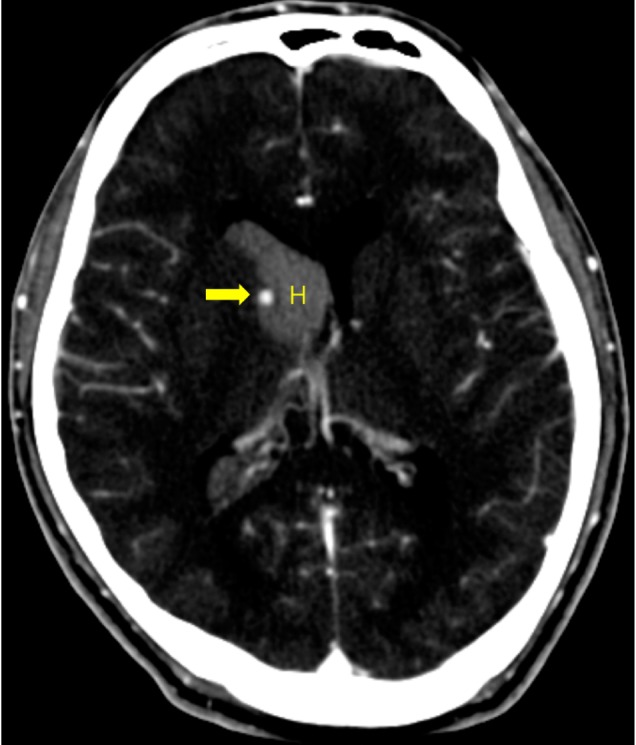
Fig. 2
Digital subtraction angiography (DSA) images. (A) Aneurysm from lenticulostriate artery is found in initial DSA. (Arrow: aneurysm, Arrowhead: lenticulostriate artery). (B) Follow-up DSA after initial clipping shows residual aneurysm with applied clips. (Arrow: aneurysm, Arrowhead: clips). (C) Final DSA shows disappeared aneurysm after resection.
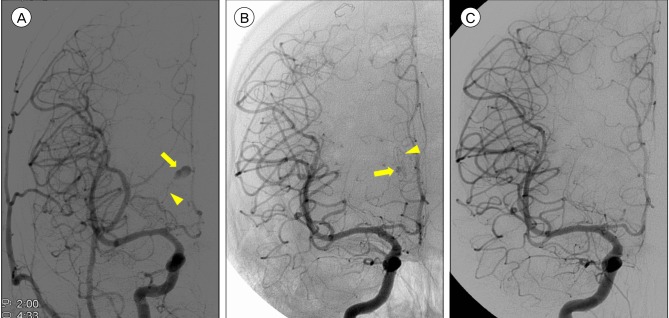
Fig. 3
Intraoperative photography. (A) Right lateral ventricle is exposed by interhemispheric approach. Saccular aneurysm sac is observed with small bleb. (Arrow: aneurysm, F: falx cerebri, CC: corpus callosum). (B) Clips are applied at the margin of caudate nucleus. (Arrow: clips, F: falx cerebri, CC: corpus callosum). (C) The head of right caudate nucleus is dissected and pseudoaneurysm is totally resected (Arrow: caudate nucleus, F: falx cerebri).
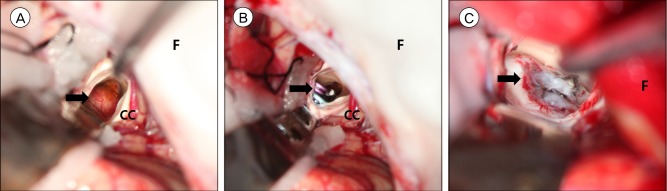
Fig. 4
Photomicrography. (A) Hematoxylin-eosin stain, original magnification ×40. (*: Recent thrombi with partial organization are observed, Arrow: partially ruptured vascular wall). (B) Elastin stain, original magnification ×200, this is magnification of yellow circle area in Fig. 4A (Black arrow: elastin layer is stained, White arrow: elastin layer is discontinued at the wall of hematoma).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download