Abstract
We report a case of spontaneous bilateral intracranial vertebral artery dissecting aneurysms with subarachnoid hemorrhage. One dissecting lesion was treated with a coronary balloon-mounted stent (BMS) technique; however, due to differences in access route tortuosity, the other lesion was treated with a self-expandable stent (SES) technique. After 2 months, the angiographic outcome showed complete healing of the dissected segment on the side that was treated with BMS; in contrast, the dissection lesion appeared to be re-growing on the side that was treated with SES. Complete treatment of the aggravated lesion was achieved by additional deployment of BMSs. Therefore, we have provided a discussion of the possible reasons for this difference in outcome according to the stent type.
Intracranial vertebral artery dissecting aneurysms (VADAs) presenting with subarachnoid hemorrhage (SAH) are rare, however, their risks for morbidity, mortality, and rebleeding are high.10)15)18) The goal of treatment for ruptured VADAs is to isolate the dissecting aneurysm from the cerebral circulation in order to prevent rebleeding.21) The rebleeding rate following SAH is high; therefore, ruptured VADAs should be treated at an early stage. The treatment options are divided into surgical and endovascular approaches. Surgical treatment options include ligation of the proximal VA and direct clipping or trapping of the aneurysm. These surgical procedures are associated with high risk of morbidity and mortality.19) Additional bypass surgery is sometimes required in order to ensure collateral circulation.
Among the numerous acceptable treatment options, several authors have reported that stent reconstruction has recently been recognized as an effective treatment for VADAs.12)17)22) In particular, in cases of bilateral VADS, overlapping multiple stent is one of the suggested treatment options.
Here, we report on a case of bilateral intracranial VADAs. Due to differing tortuosity of the access roots, multiple overlapping stents were performed with balloon-mounted coronary stents (BMSs) on one side, and self-expanding nitinol stents (SESs) on the other. Assessment of angiographic outcomes after 2 months revealed differences in follow-up results depending on stent type.
A 55-year-old female with stupor was admitted to our hospital. A computed tomography (CT) scan with CT angiography revealed thick SAH with associated hydrocephalus and fusiform dilation of bilateral V4 VAs (Fig. 1). The patient underwent cerebral angiography, which revealed the presence of a bilateral V4 segment dissecting aneurysm, and a double origin posterior inferior cerebellar artery (PICA) was observed on the left side (Fig. 2A, B). Each vertebral artery was similar in size. Because the hematoma showed even distribution on CT, the ruptured side could not be specified. We planned on simultaneous treatment of the bilateral lesions using the overlapping stent-alone technique.
The procedure was performed under local anesthesia using standard neuro-endovascular techniques. Due to the risk of rebleeding, intravenous injection of heparin was not administered at the beginning of the procedure. In order to facilitate simultaneous treatment, we opted for a transfemoral approach. On the left-sided lesion, an SES was applied because the BMS could not be advanced into the left VA due to vascular tortuosity. Two SESs (Neuroform3 stent, Boston Scientific, Voisins-le-Bretonneux, France; 4.5×20 mm) were deployed across the dissecting lesion. The guiding catheter was then repositioned at the origin of the right VA. The first BMS (Driver RX, Medtronic, Minneapolis, MN, USA; 3.5×18 mm) was deployed at the proximal end of the dissection. A second BMS (Driver RX, Medtronic, Minneapolis, MN, USA; 3.5 × 15 mm) was placed distally within the previously placed stent with a telescoping overlap of approximately 10 mm. In this fashion, the dissecting segment was covered by the 2 stents. We did not perform stent-assisted coiling because an additional coiling procedure might have risked re-rupture of the dissecting aneurysm and led to a life-threatening situation. Final images confirmed the placement of the stents across the entire length of the aneurysms (Fig. 2C). The patient received 100 mg of oral acetyl salicylic acid daily after the initial treatment. A follow-up right VA angiography 2 months after the initial treatment showed minimal contrast filling into the pseudo lumen through the stent and showed near-complete healing of the lesion (Fig. 2D). In contrast, a left VA angiogram showed an increase in size of the dissecting aneurysm (Fig. 2E). The angiographic result led us to believe that the lesion on the left side was ruptured and that the SESs used on this lesion were not sufficient to induce pseudo-luminal thrombosis.
Retreatment was performed via a transbrachial approach in order to overcome VA tortuosity. The transradial or transbrachial approach, which is the preferred approach, offers a more direct route to the ipsilateral vertebral artery in cases where the vertebral artery has a more acute angle or tortuosity.1)2)
Therefore, based on navigation of the stent into the lesion, the transbrachial method is considered superior to the transfemoral approach because the guiding catheter can be advanced distally without increasing the risk of flow arrest. Three BMSs (FlexMaster F1 stent, Abbott, Abbott Park, IL, USA) with commercially available lengths of 19 mm, 16 mm, and 12 mm and diameters of 3.5 mm were deployed using an overlapping technique (Fig. 3). Evaluation of CT angiography with maximum intensity projection on the first day after retreatment showed a marked reduction in the size of the pseudolumen. A follow-up left VA angiogram 5 months after retreatment showed complete healing of the aneurysm without in-stent stenosis (Fig. 4A-C). The patient recovered without any neurological deficits.
The goal of treatment for ruptured VADAs is to isolate the dissecting aneurysm from the cerebral circulation in order to prevent rebleeding. Treatment of VADAs with stent-assisted coil embolization or stent-alone is increasing.5)13)16) Intravascular stent placement can alter the flow dynamics within the pseudolumen and this hemodynamic change can promote permanent intra-aneurysmal thrombus formation while preserving parent arterial blood flow. This technique can be used in patients undergoing VA dissection with a hypoplastic contralateral VA, dissecting aneurysms involving the origin of the PICA, bilateral VA dissections, and fusiform dissecting aneurysms while securing adequate blood flow to the posterior circulation without the risk of an additional bypass operation. Although stent treatment provides an advantage in maintaining patency of parent VAs, it has several limitations as well. These include the risk of vessel rupture during placement of the stent, acute occlusion of the stent or thromboembolic complications because of insufficient anticoagulation and antiplatelet therapy in the acute stage of SAH, and inadequate occlusion of the pseudolumen with subsequent regrowth of the aneurysm.8)23)
Thus, the management of a bilateral VADA presenting with SAH is controversial. In some cases, successful treatment with staged bilateral VA occlusion or staged coil trapping and covered stent grafts has been reported.3)14)20) However, staged treatment techniques do not apply in all cases, and the patient must be able to tolerate balloon test occlusion. In addition, occlusion of 1 VA may increase the hemodynamic shear stress and flow in the contralateral VA. In some cases, these conditions will lead to rupture of the intact dissecting aneurysm.4) Therefore, in some cases, simultaneous performance of trapping and proximal occlusion is an alternative treatment option that is worthy of consideration. In a recent study, Park et al. reported that stent-only therapy using single, double, or triple overlapping stents was safe and effective for treatment of VBDAs.11) In addition, multiple overlapping stent therapy, but not the single stent, resulted in angiographic improvement.5) Stent application over the dissecting segment, especially with multiple overlapping stents, can lead to hemodynamic changes (decreases in inflow complexity, momentum, velocity, and wall shear stress), whereas intra-aneurysmal blood turnover time is increased.6)7)9)24) Therefore, we decided to perform simultaneous insertion of multiple overlapping stents in bilateral VADAs in order to prevent growth or rupture of the contralateral aneurysm after unilateral therapy and because we were not certain which aneurysm had ruptured.
In our case, the lesion on the right side, treated with the BMS, showed successful remodeling on follow-up angiogram with notable reduction of contrast filling in the pseudolumen. However, the lesion on the left side, treated with the SES with high porosity, was aggravated on follow-up angiogram, and a second treatment using the BMS was required, which eventually led to complete remodeling. SES has good lateral flexibility and navigability. Therefore, compared with BMS, SES can be more easily delivered to the target lesion through a tortuous cranial vessel. However, its large porosity might be insufficient for induction of intra-aneurysmal hemodynamic changes for thrombus formation. In addition, SESs have enough radial force to prevent stent migration, however, radial force may be insufficient for occlusion of the inlet or outlet of the dissecting aneurysm.20) Therefore, slow and careful balloon inflations with BMSs for prevention of iatrogenic dissection rupture may be more effective in VADs as compared with SES because balloon infarctions support more dissected wall apposition.
In our case, the following reasons might explain the regrowth dissection on the left side: first, the initial site of rupture might be the left VA, second, the stent was not fully recovered at the dissection site, and, finally, different stent types were used. Although it is difficult to conclude from only 1 case that BMS is the more effective treatment option, we believe that our treatment method may be helpful. In addition, we are of the opinion that the multiple stent technique is safer than the coil trapping technique in preserving the anterior inferior cerebellar artery (AICA), which is involved in the dissected segment.
There are several potential limitations of this case. First, the differences in follow-up angiographic results could be attributed to the fact that only 1 VA was ruptured. Indeed, BMS and SES can work differently in treatment of VADA; however, ruptured and non-ruptured VADAs should exhibit different behavior. Second, the successful angiographic result after the second session of BMS stenting may be due to either the features of BMS or just additional flow-diverting effects from add-on stenting. If SESs were used in the second session, the angiographic result might have been the same; thus, we cannot state that only BMS produces good results after a second session of stenting on the basis of only 1 case. Nevertheless, for physicians considering treatment of bilateral VADAs, especially with a multiple stent technique, BMS may be more suitable because of its lower stent porosity size and more secure vessel attachment.
Acute spontaneous bilateral vertebral artery dissecting aneurysms were treated successfully using a multiple stent technique; however, different angiographic results were obtained for each side. We believe that this was due to the differences between stent profiles used on each side. We cannot conclude from this single case that multiple BMS is superior to multiple SES in treatment of a ruptured VA dissecting aneurysm. However, in treatment of an unstable and expanding lesion after employment of a multiple stent technique with SES, we recommend BMS because it has less porosity and strong vessel attachment characteristics.
In the future, we believe that technological developments and improvements of these materials will provide even better and safer treatment options for management of VADA.
References
1. Bendok BR, Przybylo JH, Parkinson R, Hu Y, Awad IA, Batjer HH. Neuroendovascular interventions for intracranial posterior circulation disease via the transradial approach: Technical case report. Neurosurgery. 2005; 3. 56(3):E626. discussion E626. PMID: 15730593.

2. Horton TG, Kalapos P, Cockroft KM. Brachial artery approach for endovascular treatment of posterior circulation intracranial vascular disease: Technique and application in 5 cases. J Stroke Cerebrovasc Dis. 2012; 1. 21(1):68–74. PMID: 20851626.

3. Inoue A, Kohno K, Takechi A, Kohno K, Matsushige T, Takeda T. Bilateral vertebral artery dissecting aneurysm with subarachnoid hemorrhage treated with staged bilateral vertebral artery coil occlusion: A case report. Surg Neurol. 2008; 9. 70(3):319–322. discussion 322. PMID: 18207505.

4. Katsuno M, Mizunari T, Kobayashi S, Takahashi H, Teramoto A. Rupture of a vertebral artery dissecting aneurysm developing immediately after trapping of a dissecting aneurysm on the contralateral vertebral artery: Case report. Neurol Med Chir (Tokyo). 2009; 10. 49(10):468–470. PMID: 19855144.
5. Kim BM, Shin YS, Kim SH, Suh SH, Ihn YK, Kim DI, et al. Incidence and risk factors of recurrence after endovascular treatment of intracranial vertebrobasilar dissecting aneurysms. Stroke. 2011; 9. 42(9):2425–2430. PMID: 21778439.

6. Kim M, Levy EI, Meng H, Hopkins LN. Quantification of hemodynamic changes induced by virtual placement of multiple stents across a wide-necked basilar trunk aneurysm. Neurosurgery. 2007; 12. 61(6):1305–1312. discussion 1312-3. PMID: 18162911.
7. Kim M, Taulbee DB, Tremmel M, Meng H. Comparison of two stents in modifying cerebral aneurysm hemodynamics. Ann Biomed Eng. 2008; 5. 36(5):726–741. PMID: 18264766.

8. MacKay CI, Han PP, Albuquerque FC, McDougall CG. Recurrence of a vertebral artery dissecting pseudoaneurysm after successful stent-supported coil embolization: Case report. Neurosurgery. 2003; 9. 53(3):754–759. discussion 760-1. PMID: 12943592.

9. Meng H, Wang Z, Kim M, Ecker RD, Hopkins LN. Saccular aneurysms on straight and curved vessels are subject to different hemodynamics: Implications of intravascular stenting. AJNR Am J Neuroradiol. 2006; 10. 27(9):1861–1865. PMID: 17032857.
10. Mizutani T, Aruga T, Kirino T, Miki Y, Saito I, Tsuchida T. Recurrent subarachnoid hemorrhage from untreated ruptured vertebrobasilar dissecting aneurysms. Neurosurgery. 1995; 5. 36(5):905–911. discussion 912-3. PMID: 7791980.

11. Park SI, Kim BM, Kim DI, Shin YS, Suh SH, Chung EC, et al. Clinical and angiographic follow-up of stent-only therapy for acute intracranial vertebrobasilar dissecting aneurysms. AJNR Am J Neuroradiol. 2009; 8. 30(7):1351–1356. PMID: 19342544.

12. Ramgren B, Cronqvist M, Romner B, Brandt L, Holtas S, Larsson EM. Vertebrobasilar dissection with subarachnoid hemorrhage: A retrospective study of 29 patients. Neuroradiology. 2005; 2. 47(2):97–104. PMID: 15711986.

13. Sadato A, Maeda S, Hayakawa M, Kato Y, Sano H, Hirose Y, et al. Endovascular treatment of vertebral artery dissection using stents and coils: Its pitfall and technical considerations. Minim Invasive Neurosurg. 2010; 10. 53(5-6):243–249. PMID: 21302192.

14. Sakamoto S, Ohba S, Shibukawa M, Kiura Y, Okazaki T, Arita K, et al. Staged bilateral vertebral artery occlusion for ruptured dissecting aneurysms of the basilar artery: A report of 2 cases. Surg Neurol. 2005; 11. 64(5):456–461. discussion 461. PMID: 16253701.

15. Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001; 3. 344(12):898–906. PMID: 11259724.

16. Shin YS, Kim BM, Kim SH, Suh SH, Ryu CW, Koh JS, et al. Endovascular treatment of bilateral intracranial vertebral artery dissecting aneurysms presenting with subarachnoid hemorrhage. Neurosurgery. 2012; 3. 70(1 Suppl Operative):75–81. discussion 81. PMID: 21796008.

17. Sugiu K, Tokunaga K, Watanabe K, Sasahara W, Ono S, Tamiya T, et al. Emergent endovascular treatment of ruptured vertebral artery dissecting aneurysms. Neuroradiology. 2005; 2. 47(2):158–164. PMID: 15703929.

18. Yamada M, Kitahara T, Kurata A, Fujii K, Miyasaka Y. Intracranial vertebral artery dissection with subarachnoid hemorrhage: Clinical characteristics and outcomes in conservatively treated patients. J Neurosurg. 2004; 7. 101(1):25–30. PMID: 15255247.

19. Yamaura A, Watanabe Y, Saeki N. Dissecting aneurysms of the intracranial vertebral artery. J Neurosurg. 1990; 2. 72(2):183–188. PMID: 2404089.

20. Yoon SM, Shim JJ, Kim SH, Chang JC. Bilateral vertebral artery dissecting aneurysms presenting with subarachnoid hemorrhage treated by staged coil trapping and covered stents graft. J Korean Neurosurg Soc. 2012; 3. 51(3):155–159. PMID: 22639713.

21. Yoon W, Seo JJ, Kim TS, Do HM, Jayaraman MV, Marks MP. Dissection of the V4 segment of the vertebral artery: Clinicoradiologic manifestations and endovascular treatment. Eur Radiol. 2007; 4. 17(4):983–993. PMID: 16670864.

22. Yoon WK, Kim YW, Kim SR, Park IS, Kim SD, Jo KW, et al. Angiographic and clinical outcomes of stent-alone treatment for spontaneous vertebrobasilar dissecting aneurysm. Acta Neurochir (Wien). 2010; 9. 152(9):1477–1486. discussion 1486. PMID: 20508955.

23. Yuki I, Murayama Y, Vinuela F. Endovascular management of dissecting vertebrobasilar artery aneurysms in patients presenting with acute subarachnoid hemorrhage. J Neurosurg. 2005; 10. 103(4):649–655. PMID: 16266047.

24. Zenteno MA, Santos-Franco JA, Freitas-Modenesi JM, Gomez C, Murillo-Bonilla L, Aburto-Murrieta Y, et al. Use of the sole stenting technique for the management of aneurysms in the posterior circulation in a prospective series of 20 patients. J Neurosurg. 2008; 6. 108(6):1104–1118. PMID: 18518712.

Fig. 1
(A, B) Brain computed tomography (CT) shows diffuse subarachnoid hemorrhage and early hydrocephalus.
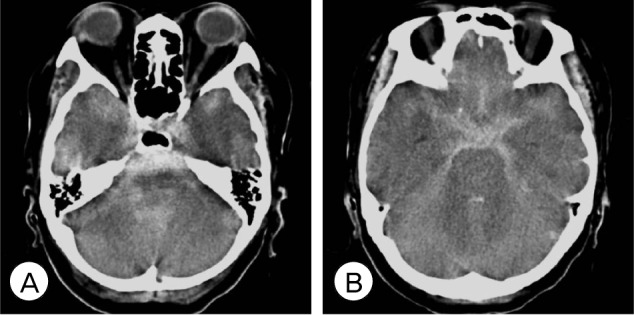
Fig. 2
(A, B) Initial angiograms show bilateral intracranial vertebral artery (VA) dissection. The risk of rupture was higher for the left VA because the size of the aneurysm was larger on the left side. Nevertheless, we were not certain about which side had experienced rupture (A: right, B: left). (C): Native image after bilateral VA treatment shows that 2 self-expanding nitinol stents (SESs) on the left VA and 2 balloon-mounted coronary stents (BMSs) on the right VA were deployed across the dissecting lesion. (D, E) Two-month follow-up angiograms show decreased but notable contrast filling to the aneurysm sac through the stent and increased aneurysm sac filling (D:right, E:left).
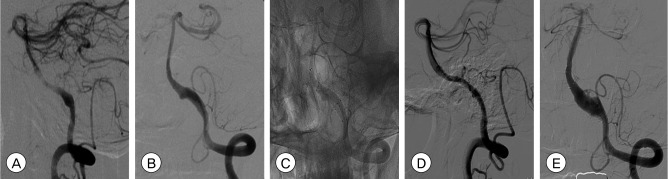
Fig. 3
Left VA native image shows the stent-within-a-stent construct created with three balloon expanding stents (FlexMaster F1 stent, Abbott, Abbott Park, IL, USA) in the left VA.

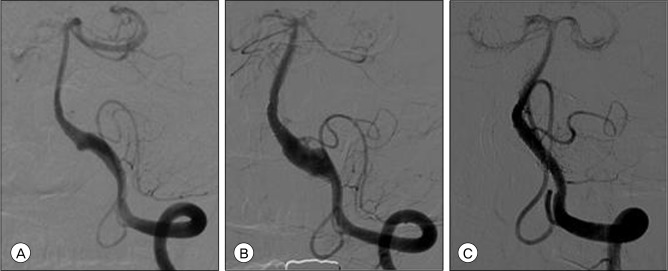
Fig. 4
Serial angiogram of the left VA. (A) Initial angiogram showing a left V4 segment dissecting aneurysm. (B) Follow-up angiogram obtained 2 months after the first treatment showing increased aneurysm sac filling. (C) Follow-up angiogram obtained 5 months after the second treatment, no in-stent stenosis and complete healing of the aneurysm and restoration of the normal caliber of the vessel is observed.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download