Abstract
Objective
Symptoms of posterior reversible encephalopathy syndrome (PRES) include headache, altered mental status, visual disturbances, and seizures. Typical radiological features include edema of the parieto-occipital lobes. The purpose of this study is to review the clinical and radiological findings in patients diagnosed with PRES.
Methods
All patients diagnosed with PRES between January 2006 and December 2012 were retrospectively included in this study. We reviewed demographic and clinical characteristics, and radiological findings.
Results
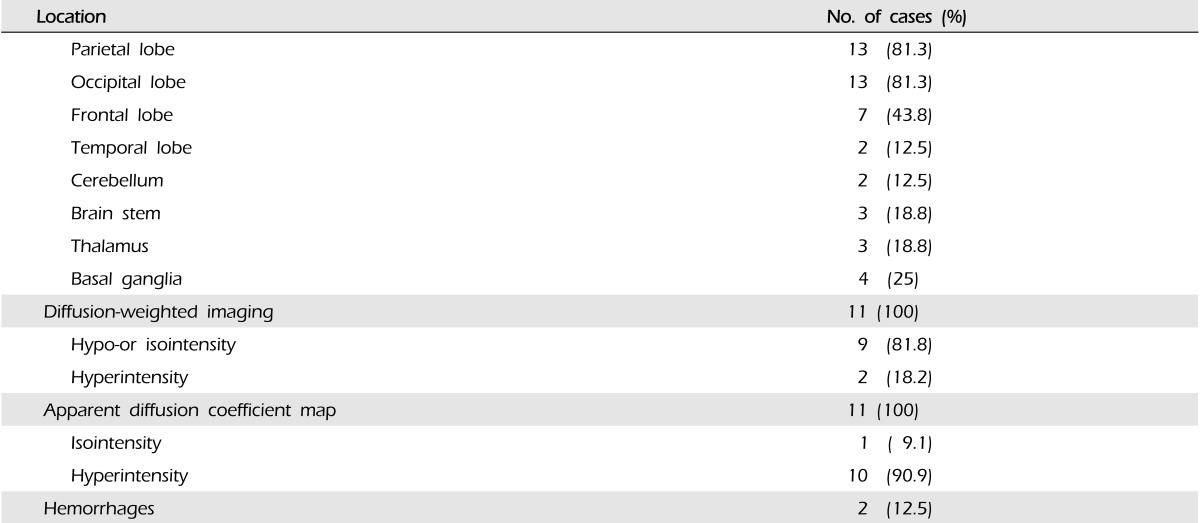
We identified 16 patients with PRES. The most common clinical presentation was seizure (n = 12, 75%). Clinical recovery occurred in all patients within days (mean, 5.7 ± 4.6 days). Comorbid conditions included hypertension (n = 4, 25%), cytotoxic medications (n = 3, 18.8%), sepsis (n = 4, 25%), malignancy (n = 4, 25%), subarachnoid hemorrhage (n = 1, 6.3%), autoimmune disorders (n = 1, 6.3%) and eclampsia (n = 1, 6.3%). The most commonly involved location was the parieto-occipital lobe (n = 13, 81.3%). Atypical radiological findings included significant basal ganglia involvement in 4 episodes; brainstem in 3, cerebellum in 2, and thalamus in 3. Eleven patients (68.8%) underwent diffusion-weighted imaging and apparent diffusion coefficient mapping. Of those, 9 patients (81.8%) had hypo- or isointensity on diffusion-weighted imaging. On the apparent diffusion coefficient map, 10 patients (90.9%) had hyperintensity, and the other had normal values.
Conclusion
We suggest that PRES may occur in patients with complex systemic conditions. The prognosis of PRES is usually benign. Physicians should be aware of certain atypical radiological findings to avoid a delayed diagnosis of PRES, as delayed diagnosis and treatment can result in permanent neurological sequlae.
Posterior reversible encephalopathy syndrome (PRES) is a clinico-radiological entity characterized by a varying combination of symptoms and signs that include impaired consciousness, seizure activity, headache, visual abnormalities, nausea/vomiting, and focal neurological deficits.16)27) This syndrome was first reported by Hinchey and colleagues in 1996.16) The syndrome is characterized by a primarily vasogenic edema of the subcortical white matter with a predilection for the parenchyma supplied by the posterior circulation, and is potentially reversible.4) However, recognition of atypical imaging findings, such as varied distribution patterns, cytotoxic edema, infarction, hemorrhage, and contrast enhancement has recently increased.15)32)
However, many patients are still not diagnosed during the initial stage. Studies have reported on precipitants of PRES, such as hypertension, acute renal failure, eclampsia, blood transfusion, infection, sepsis, radio-contrast, and immunosuppressive agents, and certain cytotoxic medications. PRES must be taken into consideration; radiological studies, particularly magnetic resonance imaging (MRI), should be performed. Without immediate diagnosis and administration of appropriate treatment, which have the greatest effect on prognosis, progression to ischemia, infarction, and death might occur.
The aim of this study is to identify the clinical and radiological findings of all patients who were admitted to our institution with a diagnosis of PRES, and to review the current literature on this syndrome.
The radiological report data bases, involving the neurosurgery, neurology, cardiovascular, oncology, rheumatology, and obstetrics departments of the author's affiliated hospitals were searched for the following items cited on brain MRI reports from January 2006 to December 2012: PRES, posterior reversible encephalopathy, posterior reversible leukoencephalopathy, preeclampsia and eclampsia, toxemia of pregnancy, hypertensive encephalopathy, and hypertensive crisis. Patients included in this study had a clinical presentations and specific radiological abnormalities consistent with PRES.
The following criteria were used in final diagnosis of PRES: typical clinical presentation of PRES, including headaches, visual disturbance, altered mental functioning, and seizures with an underlying etiology such as hypertension, drug toxicity. Also included were other common causes, like renal involvement with renal insufficiency and infection supported by imaging of brain parenchyma that demonstrated signal changes on T2 weighted images and fluid-attenuated inversion recovery (FLAIR) images as well as apparent diffusion coefficient (ADC) values.
We collected data on demographics, predisposing conditions, presenting symptoms, co-morbidities, blood pressure measurements at the initial stage and time to clinical recovery.
All neuroimages (including brain computed tomographs [CT] and MRIs) were reviewed by study neuroradiologists for identification of various anatomical regions and atypical features of PRES (Fig. 1).
We identified 16 patients (10 females and 6 males). Mean age at presentation was 47 ± 21.6 years (range, 12-83). The most common clinical presentation was seizure, observed in 12 patients (75%) including 10 patients (83.3%) with generalized tonic-clonic seizures, and 2 patients (16.7%) with partial seizures. Other clinical presentations included decreased consciousness in 3 patients (18.8%), severe headache in 4 (25%), and visual disturbances in 2 (12.5%). Comorbid conditions included hypertension (n = 4, 25%), cytotoxic medications (n = 3, 18.8%), sepsis (n = 4, 25%), malignancy (n = 4, 25%), subarachnoid hemorrhage (n = 1, 6.3%), autoimmune disorders (n = 1, 6.3%), and eclampsia (n = 1, 6.3%). Thirteen patients (81.3%) had acute hypertension. Of these, 4 patients had known preexisting hypertension. Mean peak systolic blood pressure at symptom onset was 159.7 (range, 120-200) and diastolic was 94.6 mmHg (range, 80-130). Malignancy was present in 4 (25%) patients and included invasive ductal carcinoma, metastatic carcinoma, urethral adenocarcinoma, and cecal adenocarcinoma. Two of these patients had received chemotherapy. One of these 2 patients had urethral adenocarcinoma treated with cisplatin; the other had cecal adenocarcinoma treated with 5-fluoruracil/oxaliplatin. The patient with Behcet disease and pemphigus vulgaris was treated with cyclosporine-A.
All patients underwent initial neuroimaging with either brain CT scan (n = 5) or MRI (n = 11). Several topographic MR imaging patterns of PRES have been described (Fig. 1).4) Bilateral cerebral subcortical white matter involvement was observed in all patients. Parietal lobe involvement was observed in 13 (81.3%) patients, occipital in 13 (81.3%), frontal in 7 (43.8%), and temporal in 2 (12.5%). The cerebellum was involved in 2 (12.5%) patients, brain stem in 3 (18.8%), thalamus in 3 (18.8%), and basal ganglia in 4 (25%). Eleven patients (68.8%) underwent diffusion-weighted imaging (DWI) and ADC mapping. Among them, 9 patients (81.8%) had hypo- or isointensity on DWI. On the ADC map, 10 patients (90.9%) had hyperintensity, and the other had normal values. Two patients had intracranial hemorrhage. The radiological spectrum of lesion distribution of 16 patients with PRES was described in Table 2.
We performed symptomatic treatment and control of the causative factors. Corrections of severe hypertension, treatment of seizure, and removal or reduction of causative medications are mandatory. Hypertension was treated with intravenous antihypertensive drug, usually nicardipine or labetalol. In patients with presenting seizure or decreased consciousness were treated antiepileptic drugs. We usually prefer to use intravenous antiepileptic drugs (benzodiazepines, valproate or levetiracetam).
There were 2 patients with PRES related to chemotherapy. One of these patients had urethral adenocarcinoma treated with cisplatin. She developed seizures after 3 cycles of chemotherapy. Following administration of antiepileptic and antihypertensive drugs, her neurologic symptoms recovered fully 3 days later. After dose reduction of cisplatin, she tolerated the remaining cycles of chemotherapy.
In the patient with Behcet disease and pemphigus vulgaris, cyclosporine-A was administered for 6 days before the onset of neurologic symptoms. Her symptoms improved 1 week after the control of hypertension and discontinuation of cyclosporine-A (case 16).
Clinical recovery occurred within a mean duration of 5.7 ± 4.6 days (range, 2-15). Clinical characteristics in 16 patients with PRES are described in Table 1.
A unique pattern of brain vasogenic edema seen in the setting of neurotoxicity has been recognized as PRES associated with eclampsia, cyclosporine after organ transplantation, and severe hypertension.35) However, the mechanism involved in development of PRES is uncertain. Although several theories are proposed, the one having the widest acceptance suggests that rapid development of hypertension results in malfunction of cerebral autoregulation, with the posterior head region being particularly affected (where fewer sympathetic innervations occur), followed by hyperperfusion with extravasation of protein and fluid, resulting in development of focal vasogenic edema.16)26)30) An alternative theory, associated primarily with preeclampsia, eclampsia, and sepsis, suggests involvement of endothelial dysfunction.2)10) Another theory implicates vasospasm with subsequent ischemia.23)33)
PRES without hypertension is reported in 20-40% of patients.4-6) In the majority of cases, although hypertension (moderate to severe) is present, reported blood pressure at neurotoxicity does not reach the limit of autoregulation.3)4) In addition, several recent studies report that patients with severe hypertension showed less vasogenic edema than normotensive patients. This result would be unexpected if the mechanism of PRES was severe hypertension with failed autoregulation.3)5) The majority of our patients (82.3%) showed acute elevation of blood pressure.
Considering the common major conditions associated with development of PRES, such as transplantation, autoimmune disease, preeclampsia/eclampsia, cancer chemotherapy, infection and sepsis, some degree of endothelial injury appears to be a consistent finding.4)10) In our study, PRES was observed in association with a wide range of disorders and predisposing conditions, ranging from hypertension, eclampsia to sepsis, subarachnoid hemorrhage, and exposure to cytotoxic drugs as well as Behcet diseases.
Association of high-dose multidrug cytotoxic chemotherapy with PRES, particularly in children with leukemia, has also been reported.9) In additionally, PRES has been reported in association with the following chemotherapeutic drugs: cytarabine, cisplatin, gemcitabine, and bevacizumab.14)17) Symptoms may occur over a period of several days although may only be observed in an acute setting. In our series, 2 patients received chemotherapy for malignancy. One of these patients had urethral adenocarcinoma treated with cisplatin. After dose reduction of cisplatin, she tolerated the remaining cycles of chemotherapy. The patient with Bechet disease and pemphigus vulgaris was treated with cyclosporine-A. Her symptoms improved 1 week after the control of hypertension and discontinuation of cyclosporine-A (case 16) (Fig. 2). Cyclosporine-A can affect vascular endothelium, cause disruption of the blood-brain barrier, and increase arterial blood pressure.21)29) When such complications occur, the offending drug should be withdrawn immediately.
Generalized seizure is a common occurrence in patients with PRES (80-90%).16)20) Seizure is related to the location of the focal brain abnormality that provokes an epileptic focus, because locations associated with PRES occur most often in parieto-occipital lobes.
On radiological finding, PRES typically presents as vasogenic edema, most often involving the posterior white matter of the cerebral hemispheres, particularly the bilateral parieto-occipital lobes, usually without involvement of the calcarine and paramedian occipital-lobe.16) DWI can be helpful in identification of cytotoxic or vasogenic edema and evaluation of the potential for occurrence of complications such as ischemia or infarction.1)13)18)28) Vasogenic edema typically shows iso or hypointensity in DWI and hyperintensity on the ADC map, and cytotoxic edema shows hyperintensity on DWI and hypointensity on the ADC map.24)29) In our series, 11 patients (68.8%) underwent DWI and ADC mapping. Among them, 9 patients (81.8%) had hypo- or isointensity on DWI. On the ADC map, 10 patients (90.9%) had hyperintensity, and the other had normal values. This pattern is consistent with vasogenic edema. Although the parietal and occipital lobes are primarily involved, other cerebral structures are often affected. Atypical manifestations include brainstem variant or involvement of the frontal lobes, basal ganglia, brainstem, and deep cerebral white matter, with minimal high signal intensity on the parieto-occipital lobes (Fig. 1).1)19) However, frequent frontal lobe involvement, rather than atypical localization, has recently been described. Therefore, the hypothesis of less sympathetic innervation of arterioles supplied by the vertebrobasilary system, compared with the anterior circulation, which presumably protects the brain from sudden significant increases in intravascular pressure, is not completely accurate.12) We frequently observed these atypical imaging presentations in our patients, especially in those with frontal lobe involvement, observed in 43.8% of patients.
Treatment of elevated blood pressure is regarded as being essential in management of PRES. The general goal of treatment is to decrease the mean blood pressure to that of premorbid levels. A neurocritical care unit, which allows for close consultation with other specialties according to the underlying systemic condition, is considered the best setting for management of PRES. In treatment of PRES, there are several important considerations: (1) elimination or reduction of the causative drug, (2) aggressive management of blood pressure in patients with hypertension, (3) treatment of seizures and status epilepticus, and (4) possible delivery by cesarean section for pregnant women who exhibit refractory symptoms.31) Prompt administration of adequate treatment will usually result in complete reversal of PRES within several days to several weeks.16)20) In our studies, the mean clinical recovery period was 5.7 ± 4.6 days (range, 2-15).
Of particular importance, reversal of PRES does not occur spontaneously. In addition, delayed diagnosis and treatment may result in permanent damage to affected areas of the brain.8) Ischemia is a major complication of PRES, and characteristically occurs in the posterior border zone between the territories of the middle and posterior cerebral arteries.8) Delay of diagnosis and treatment can result in permanent damage to affected brain tissues.
Several limitations of our study should be considered, most of which are inherent to retrospective studies in general. Patient enrollment was initially based on a search of radiology reports; therefore, some cases may have been missed.
We suggest that PRES can occur in patients with complex systemic conditions. The prognosis of PRES is usually benign. Although it is a well-known condition among neuroradiologists, many physicians working in critical areas are still not familiar with PRES. Physicians should be aware of atypical radiological findings to avoid a delayed diagnosis of PRES, as delayed diagnosis and treatment can result in permanent neurological sequlae.
References
1. Ahn KJ, You WJ, Jeong SL, Lee JW, Kim BS, Lee JH, et al. Atypical manifestations of reversible posterior leukoencephalopathy syndrome: Findings on diffusion imaging and ADC mapping. Neuroradiology. 2004; 12. 46(12):978–983. PMID: 15536557.

2. Aird WC. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood. 2003; 5. 15. 101(10):3765–3777. PMID: 12543869.

3. Bartynski WS, Boardman JF. Catheter angiography, MR angiography, and MR perfusion in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol. 2008; 3. 29(3):447–455. PMID: 18079186.

4. Bartynski WS, Boardman JF. Distinct imaging patterns and lesion distribution in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol. 2007; 8. 28(7):1320–1327. PMID: 17698535.

5. Bartynski WS, Boardman JF, Zeigler ZR, Shadduck RK, Lister J. Posterior reversible encephalopathy syndrome in infection, sepsis, and shock. AJNR Am J Neuroradiol. 2006; Nov-Dec. 27(10):2179–2190. PMID: 17110690.
6. Bartynski WS, Zeigler Z, Spearman MP, Lin L, Shadduck RK, Lister J. Etiology of cortical and white matter lesions in cyclosporin-A and FK-506 neurotoxicity. AJNR Am J Neuroradiol. 2001; Nov-Dec. 22(10):1901–1914. PMID: 11733324.
7. Bhatt A, Farooq MU, Majid A, Kassab M. Chemotherapy-related posterior reversible leukoencephalopathy syndrome. Nat Clin Pract Neurol. 2009; 3. 5(3):163–169. PMID: 19262592.

8. Casey SO, McKinney A, Teksam M, Liu H, Truwit CL. CT perfusion imaging in the management of posterior reversible encephalopathy. Neuroradiology. 2004; 4. 46(4):272–276. PMID: 15045493.

9. Cooney MJ, Bradley WG, Symko SC, Patel ST, Groncy PK. Hypertensive encephalopathy: Complication in children treated for myeloproliferative disorders- Report of three cases. Radiology. 2000; 3. 214(3):711–716. PMID: 10715035.
10. Dekker GA, Sibai BM. Etiology and pathogenesis of preeclampsia: Current concepts. Am J Obstet Gynecol. 1998; 11. 179(5):1359–1375. PMID: 9822529.

11. Domínguez-Fuentes B, Garcia-Gil D, Romero-Palacios A, Sanchez-Crespo JM, Garcia-Arjona R, Navarro-Navarro J. [Posterior reversible leukoencephalopathy in a patient with postpartum eclampsia]. Med Intensiva. 2008; 10. 32(7):361–363. Spanish. PMID: 18842228.
12. Edvinsson L, Owman C, Sjoberg NO. Autonomic nerves, mast cells, and amine receptors in human brain vessels. A histochemical and pharmacological study. Brain Res. 1976; 10. 115(3):377–393. PMID: 184880.

13. Eichler FS, Wang P, Wityk RJ, Beauchamp NJ Jr, Barker PB. Diffuse metabolic abnormalities in reversible posterior leukoencephalopathy syndrome. AJNR Am J Neuroradiol. 2002; 5. 23(5):833–837. PMID: 12006287.
14. Glusker P, Recht L, Lane B. Reversible posterior leukoencephalopathy syndrome and bevacizumab. N Engl J Med. 2006; 3. 354(9):980–982. discussion 980-2. PMID: 16510760.

15. Hefzy HM, Bartynski WS, Boardman JF, Lacomis D. Hemorrhage in posterior reversible encephalopathy syndrome: Imaging and clinical features. AJNR Am J Neuroradiol. 2009; 8. 30(7):1371–1379. PMID: 19386731.

16. Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996; 2. 334(8):494–500. PMID: 8559202.

17. Ito Y, Arahata Y, Goto Y, Hirayama M, Nagamutsu M, Yasuda T, et al. Cisplatin neurotoxicity presenting as reversible posterior leukoencephalopathy syndrome. AJNR Am J Neuroradiol. 1998; 3. 19(3):415–417. PMID: 9541291.
18. Kinoshita T, Moritani T, Shrier DA, Hiwatashi A, Wang HZ, Numaguchi Y, et al. Diffusion-weighted MR imaging of posterior reversible leukoencephalopathy syndrome: A pictorial essay. Clin Imaging. 2003; Sep-Oct. 27(5):307–315. PMID: 12932680.
19. Kitaguchi H, Tomimoto H, Miki Y, Yamamoto A, Terada K, Satoi H, et al. A brainstem variant of reversible posterior leukoencephalopathy syndrome. Neuroradiology. 2005; 9. 47(9):652–656. PMID: 15947925.

20. Kwon S, Koo J, Lee S. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Pediatr Neurol. 2001; 5. 24(5):361–364. PMID: 11516610.

21. Lassila M, Santisteban J, Finckenberg P, Salmenpera P, Riutta A, Moilanen E, et al. Vascular changes in cyclosporine A-induced hypertension and nephrotoxicity in spontaneously hypertensive rats on high-sodium diet. J Physiol Pharmacol. 2001; 3. 52(1):21–38. PMID: 11321510.
22. Lee VH, Wijdicks EF, Manno EM, Rabinstein AA. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008; 2. 65(2):205–210. PMID: 18268188.

23. Lin JT, Wang SJ, Fuh JL, Hsiao LT, Lirng JF, Chen PM. Prolonged reversible vasospasm in cyclosporin A-induced encephalopathy. AJNR Am J Neuroradiol. 2003; 1. 24(1):102–104. PMID: 12533334.
24. Min L, Zwerling J, Ocava LC, Chen IH, Putterman C. Reversible posterior leukoencephalopathy in connective tissue diseases. Semin Arthritis Rheum. 2006; 6. 35(6):388–395. PMID: 16765716.

25. Prasad N, Gulati S, Gupta RK, Kumar R, Sharma K, Sharma RK. Is reversible posterior leukoencephalopathy with severe hypertension completely reversible in all patients? Pediatr Nephrol. 2003; 11. 18(11):1161–1166. PMID: 14505162.

26. Primavera A, Audenino D, Mavilio N, Cocito L. Reversible posterior leucoencephalopathy syndrome in systemic lupus and vasculitis. Ann Rheum Dis. 2001; 5. 60(5):534–537. PMID: 11302882.

27. Pula JH, Eggenberger E. Posterior reversible encephalopathy syndrome. Curr Opin Ophthalmol. 2008; 11. 19(6):479–484. PMID: 18854692.

28. Schaefer PW, Gonzalez RG, Hunter G, Wang B, Koroshetz WJ, Schwamm LH. Diagnostic value of apparent diffusion coefficient hyperintensity in selected patients with acute neurologic deficits. J Neuroimaging. 2001; 10. 11(4):369–380. PMID: 11677876.

29. Schwartz RB, Bravo SM, Klufas RA, Hsu L, Barnes PD, Robson CD, et al. Cyclosporine neurotoxicity and its relationship to hypertensive encephalopathy: CT and MR findings in 16 cases. AJR Am J Roentgenol. 1995; 9. 165(3):627–631. PMID: 7645483.

30. Schwartz RB, Jones KM, Kalina P, Bajakian RL, Mantello MT, Garada B, et al. Hypertensive encephalopathy: Findings on CT, MR imaging, and SPECT imaging in 14 cases. AJR Am J Roentgenol. 1992; 8. 159(2):379–383. PMID: 1632361.

31. Servillo G, Bifulco F, De Robertis E, Piazza O, Striano P, Tortora F, et al. Posterior reversible encephalopathy syndrome in intensive care medicine. Intensive Care Med. 2007; 2. 33(2):230–236. PMID: 17119920.

32. Sharma A, Whitesell RT, Moran KJ. Imaging pattern of intracranial hemorrhage in the setting of posterior reversible encephalopathy syndrome. Neuroradiology. 2010; 10. 52(10):855–863. PMID: 19956935.

33. Trommer BL, Homer D, Mikhael MA. Cerebral vasospasm and eclampsia. Stroke. 1988; 3. 19(3):326–329. PMID: 3354016.

34. Vieillot S, Pouessel D, de Champfleur NM, Becht C, Culine S. Reversible posterior leukoencephalopathy syndrome after carboplatin therapy. Ann Oncol. 2007; 3. 18(3):608–609. PMID: 17164233.

35. Weingarten K, Barbut D, Filippi C, Zimmerman RD. Acute hypertensive encephalopathy: Findings on spin-echo and gradient-echo MR imaging. AJR Am J Roentgenol. 1994; 3. 162(3):665–670. PMID: 8109519.

Fig. 1
Axial fluid-attenuated inversion recovery magnetic resonance imaging (MRI) of posterior reversible encephalopathy syndrome (PRES) shows the involvement of cerebellum (A), cortex (B), frontal lobe (C), brainstem with atypical pattern (D), and thalamus/basal ganglia (E, F).
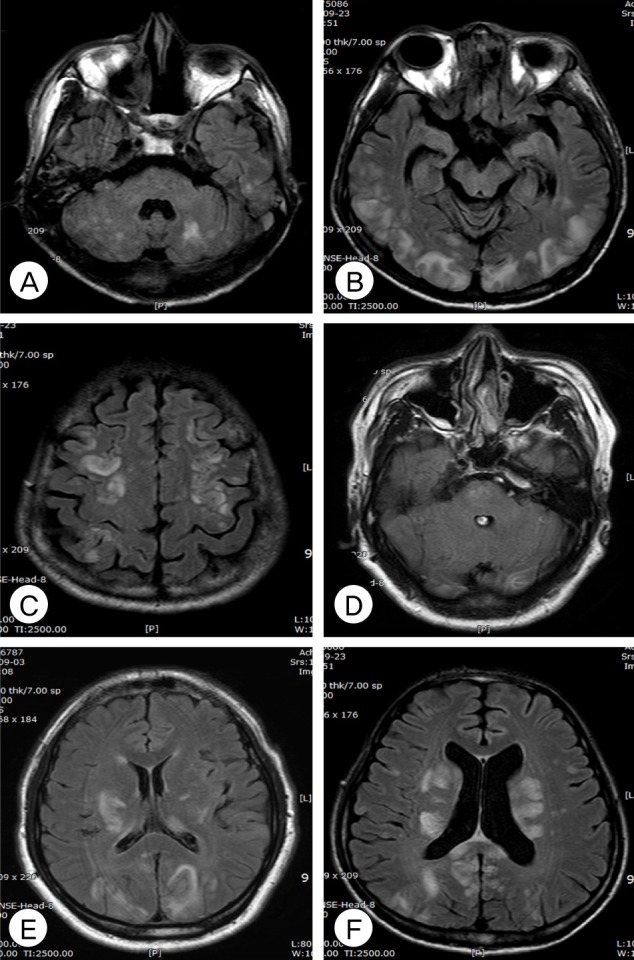
Fig. 2
A 43-year old female treated with cyclosporine-A (Patient 16). An axial computed tomography scan at onset shows low density at parieto-occipital lobes (A). Axial magnetic resonance imaging (MRI) shows a diffuse high-signal intensity lesion on fluid-attenuated inversion recovery MRI (B, C) and the apparent diffusion coefficient map (D, E) at the bilateral parieto-occipital lobes and basal ganglia. Diffusion weighted imaging shows lesions with iso and hypointensity (F) in the same distribution.
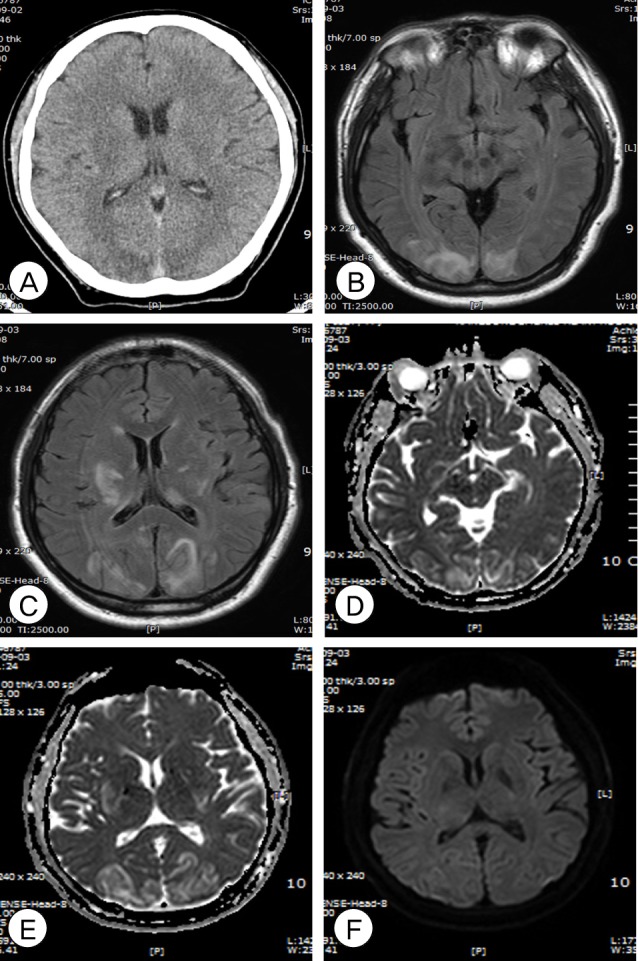
Table 1
Demographics and clinical characteristics of 16 patients with PRES
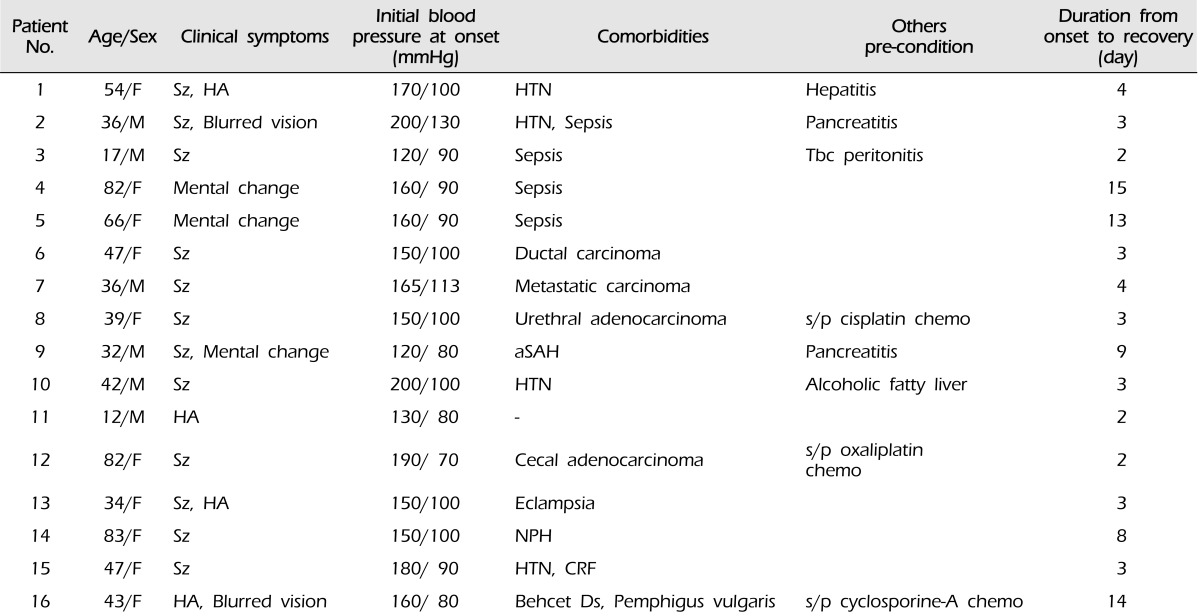
chemo= chemotherapeutic medications; CRF= chronic renal failure; Ds= disease; F= female; HA= headache; HTN= hypertension; M= male; NPH= normal pressure hydrocephalus; PRES= posterior reversible encephalopathy syndrome; aSAH= aneurysm subarachnoid hemorrhage; s/p= status post; Sz= seizure; Tbc= tuberculosis.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download