Abstract
Churg-Strauss syndrome (CSS) is a systemic necrotizing vasculitis of the small and medium vessels, associated with extravascular eosinophilic granulomas, peripheral eosinophilia, and asthma. The exact etiology of CSS is unknown. This syndrome commonly affects the lungs, peripheral nerves, skin, heart, and gastrointestinal tract, but rarely the central nervous system. Subarachnoid and intracerebral hemorrhage in CSS patients is extremely rare; however, clinicians should consider that CSS may be a cause of intracranial hemorrhage and its high rate of mortality and morbidity. The authors report on two cases of subarachnoid and intracerebral hemorrhage with CSS and discuss a brief review of CSS.
Churg-Strauss syndrome (CSS) is a systemic disorder characterized by asthma, transient pulmonary infiltrates, hypereosinophilia, and systemic vasculitis. Necrotizing eosinophilic vasculitis of medium- to small-sized blood vessels in multiple organ systems commonly affects the lungs, heart, skin, gastrointestinal tract, and nervous system. Neurological involvement is the second most common manifestation (78% of patients), usually manifesting as peripheral neuropathy.4) Cerebral hemorrhage and infarction may also occur and are important causes of death.7) CSS presenting with hemorrhagic stroke is extremely rare. Herein, we report on subarachnoid hemorrhage (SAH) presenting with ruptured intracranial vertebral artery dissection (VAD) and spontaneous intracerebral hemorrhage (ICH) affected by CSS.
A 39-year-old male patient was admitted to our hospital with complaints of bronchial asthma, rhinitis complicated by nasal polyposis, and eosinophilia in the previous seven months. He was treated with an inhalation corticosteroid, a long-acting β-stimulant, and an oral pranlukast for the asthma. He had no history of smoking or hypertension. Two months later, he suffered from diffuse arthralgia, persistent slight fever, and weight loss. Erythematosus vesiculopurpura skin rash on the dorsal aspect of the both lower leg was observed on the general physical examination.
Laboratory tests on admission to our hospital showed the following results: hematuria (trace) with proteinuria (76.2 mg/dl) (normal range 1.0-14.0 mg/dl), leukocytosis (18,800 per µl) (normal range 3,900-9,700 per µl), increased eosinophil count (3,572 per µl, 19%) (normal range 50-500 per µl, 0-10%), slightly increased Erythrocyte Sedimentation Rate (ESR) (43 mm/h) (normal range 0-10 mm/h), increased C-reactive protein (CRP) (5.41 mg/dl) (normal range 0.02-0.8 mg/dl), increased Blood Urea Nitrogen (BUN) (44.7 mg/dl) (normal range 8-25 mg/dl), and increased serum creatinine (2.5 mg/dl) (normal range 0.5-1.4 mg/dl). Other examinations revealed elevation of the eosinophil cationic protein (ECP) (38.6 µg/L) (normal range 0-13.5 µg/L). Myeloperoxidase anti-neutrophil cytoplasmic antibody (MPO-ANCA) levels were elevated at 4.0 AI (positive ≥ 1.0 AI), and negative results were observed for anti-nuclear, anti-extractable nuclear, anti-DNA, and anti-phospholipid.
Skin biopsy of the erythematosus showed leukocytoclastic vasculitis.
Chest computed tomography (CT) showed mild bronchial wall thickening in the lower lung zone and a small amount of pericardial effusion without parenchymal or interstitial infiltrates. The electrocardiogram showed normal findings, whereas the echocardiogram revealed small pericardial effusion.
Findings on abdominal CT revealed mild hepatomegaly and periportal edema with multifocal low density in the liver, more likely related to hepatic congestion or ischemia, and multiple wedge shaped low densities in both kidneys, which was diffuse involvement of nephritis. Pathological examination by kidney needle biopsy showed focal segmental glomerulosclerosis with focal and segmental mesangial Immunoglobulin A (IgA) deposits.
Based on clinical and laboratory findings, a diagnosis of CSS was made, according to the American College of Rheumatology Criteria.9) Thus, the patient received treatment with three-day intravenous (IV) methylprednisolone (1 mg/kg/day) and IV cyclophosphamide, followed by daily oral prednisone 1 mg/kg.
Over the preceding six months, the patient had exhibited noticeable numbness of the left hand. Findings of a sensory nerve conduction study revealed low amplitude in the left median nerve. Therefore, it was determined that the numbness was caused by peripheral neuropathy of the left median nerve.
On day 12 after admission, the patient exhibited sudden onset of consciousness disturbance with generalized tonic clonic seizures. Findings on brain CT showed diffuse SAH with intraventricular hemorrhage on the fourth ventricle and acute obstructive hydrocephalus (Fig. 1A). And findings on brain CT angiography (CTA) were suggestive of VAD (Fig. 1B). The patient's neurological status had worsened to Hunt-Hess Grade 4. Just after the brain CTA, we performed an emergent external ventricular drainage (EVD) catheter insertion into the right lateral ventricle through the supra-orbital.
An emergent digital subtraction angiography (DSA) immediately showed the left intracranial VAD. Extravasation of the contrast media from the dissected segment was visible on the left vertebral angiogram (Fig. 2A).
To obstruct the burst in the dissected segment, we decided to immediately occlude the VAD with coil embolization. Because of the hypoplasia of the contra-lateral VA, the trapping in the dissected segment cannot be considered for preservation of blood flow of the posterior circulation. Therefore, we planned performance of stent-assisted coiling, followed by use of a stent-within-a stent technique. Because the patient was in the acute stage of SAH, no pretreatment antiplatelet therapy was administered.
A 6F guiding catheter (Envoy; Cordis, Miami Lakes, FL) was placed at the cervical portion of the left VA (V2 segment). We placed a microcatheter (Prowler Select Plus, Cordis, Miami Lakes, FL) into the distal portion of the dissected segment for stenting, and another microcatheter (Excelsior SL-10, Boston Scientific, Natick, MA) was placed into the dissected segment of the VA for occlusion of the segment. Following insertion of four ultrasoft helical Guglielmi detachable coils (total length 41 cm) into the dissected segment, a 4.5 × 28 mm Enterprise stent was then placed through the dissected segment. A second 4.5 × 28 mm Enterprise stent was inserted using a stent-within-a-stent technique in order to support the fragile dissected vessel wall and to a induce flow diversion effect. Findings on postembolization angiography revealed complete occlusion of the segment of extravasation and preservation of the blood flow on the lesion side (Fig. 2B). Postoperatively, loading doses of aspirin (200 mg) and clopidogrel (300 mg) were given to the patient via a nasogastric tube, and the dual anti-platelets (aspirin 100 mg and clopidogrel 75 mg per day) were maintained from 24 hours after treatment. Due to his poor condition, the patient died on five days after treatment.
A 46-year-old female patient with a 10-year history of allergic rhinitis was admitted to our hospital with complaints of severe left leg pain and weakness. She had been treated intermittently with an inhalation corticosteroid for the allergic rhinitis. Lumbar magnetic resonance imaging (MRI) revealed a herniated lumbar disc between L4 and L5.
Laboratory tests on admission showed the following results: leukocytosis (18,600 per µl) (normal range 3,900-9,700 per µl), increased eosinophil count (9,411 per µl, 50.6%) (normal range 50-500 per µl, 0-10%), increased ESR (72 mm/h) (normal range 0-10 mm/h), and slightly increased CRP (1.81 mg/dl) (normal range 0.02-0.8 mg/dl). Other examinations revealed elevated ECP (> 200 µg/L) (normal range 0-13.5 µg/L) and a negative result for the parasite test. Following intravenous injection of 125 mg methylprednisolone for treatment of hypereosinophilia, operation for lumbar ruptured disc was performed. Postoperatively, the patient showed improvement of leg pain on the visual analogue scale (VAS) score for leg pain, from 7 to 2.
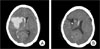
On day two after lumbar disc surgery, the patient exhibited sudden onset of headache, left side weakness and drowsy mental status. Findings on brain CT revealed ICH of approximately 60 cc in the right basal ganglia (Fig. 2A).
At that time, laboratory examinations showed leukocytosis (12,000 per µl) (normal range 3,900-9,700 per µl). MPO-ANCA levels were elevated at 8.0 AI (positive ≥ 1.0 AI) and negative results were obtained for anti-nuclear, anti-extractable nuclear, and anti-ds DNA. Sputum differential cell count showed hypereosinophilia (22%). The electrocardiogram was normal.
Based on her clinical and laboratory findings of peripheral blood hypereosinophilia, paranasal sinus abnormality, eosinophilia in the sputum (22%), and MPO-ANCA (+), a diagnosis of CSS was made. A DSA immediately showed no abnormal vascular structure. Because of the patient's neurological deterioration and a large amount of ICH, emergency stereotactic burr-hole aspiration using a navigation system (OASSTS, Medtronic, CO, USA) was performed. On day three after surgery, follow-up CT scan revealed reduction of the ICH (Fig. 2B), and on day four after surgery, the catheter was removed from the patient. At 16 days after treatment, the patient was transferred to the Department of Rehabilitation with a Glasgow Outcome Scale (GOS) score of 3.
During more than one year of follow up, the patient was treated first with oral prednisolone, and then changed to deflazacort. Follow up blood tests showed a normal peripheral eosinophil count (100 per µl, 1.4%) (normal range 50-500 per µl). The patient's neurological status was much improved, so as to be able to walk with support and the patient's GOS score improved to 4.
CCS was first described in 1951 by Churg and Strauss as a disorder of asthma, eosinophilic inflammation with systemic vasculitis involving various organ systems.3) The American College of Rheumatology (ACR) classification criteria are the most commonly used diagnostic criteria.9) The ACR criteria include both clinical and pathological features, and, for diagnosis of CSS, at least four of the following six features should be present: a history of asthma, eosinophilia, neuropathy, pulmonary infiltrates, paranasal sinus abnormality, and eosinophilic vasculitis. Diagnosis of CCS can be difficult, because the syndrome may arise at first as asthma and allergic rhinitis, and the asthma may be associated with sinusitis, occasional pulmonary infiltration, and eosinophilia. The differential diagnosis of CCS includes acute eosinophilic pneumonia, chronic eosinophilic pneumonia, histiocytosis X, eosinophilic granuloma, and Wegener's granulomatosis. Pathological confirmation of eosinophil-rich inflammatory infiltrates with granuloma formation in connective tissues and blood-vessel walls is important.
CSS has been divided into three clinical phases: prodromal, eosinophilic, and vasculitic phase, which may or may not be sequential. The prodromal phase is characterized by asthma and allergic rhinitis. The eosinophilic phase is marked by peripheral eosinophilia, eosinophilic tissue inflammation in the lungs, gastrointestinal tract, and skin. The vasculitic phase involves constitutional symptoms, including fever, myalgias, and weight loss. This phase involves various organs: the lungs, heart, peripheral nervous system, kidney, lymph nodes, muscle, and skin.16) In our two present cases, the clinicopathologic findings of Case 1 were compatible with the vasculitic phase, and those of Case 2 were compatible with the eosinophilic phase.
Peripheral neuropathy is the second most common manifestation (65-75%).4) Mononeuritis multiplex is the most frequent finding.4) The peroneal nerve is commonly involved, however, the ulnar, radial, median and tibial nerves may also be involved.6) In our Case 1, results of sensory nerve conduction study showed low amplitude in the left median nerve, indicating peripheral neuropathy of the left median nerve.
Although the overall prognosis for CSS is generally good, diffuse organ involvement such as the cardiovascular system and/or Central Nervous System (CNS), is suggestive of a poor prognosis.14) CNS involvement may include palsies of the cranial nerves (ischemic optic neuritis), cerebral hemorrhage or infarction, convulsion, coma, and psychosis. Cerebral hemorrhage and infarction are also important causes of death. Despite treatment, neurological sequelae do not often show complete resolution.7)16)
According to reviews of the literature, intracranial hemorrhage in association with Churg-Strauss syndrome has rarely been reported (Table 1).1)2)5)8)10-13)15)18)19) SAH has been documented in a few patients in CSS.1)18)19) It is believed that neutrophils may cause damage to normal tissue, which can result in development of vasculitis. Infiltration of inflammatory cells, especially neutrophils and macrophages, has been observed in progression of vasculitis. Our Case 1 is the first reported case of a SAH in association with CSS in Korea. SAH associated with CSS is caused by vasculitic involvement of major arteries or by rupture of the dissecting aneurysm.
Change et al.2) reported the first case of pathologically documented vasculitis involving the choroid plexus causing massive intraventricular and SAH in CSS. Mencacci et al.10), who reported on a case of basal ganglia hemorrhage in association with CSS, emphasized that CSS vasculitis should be considered in the differential diagnosis of ICH. In Korea, Nam et al.12) described the first case of CSS complicated by ICH and suggested that, because cerebral hemorrhage is a major cause of morbidity and death in patients with CSS, suspicion of CSS should be important in ICH patients with a history of asthma. Our Case 2 is the second case in Korea complicated by spontaneous ICH in a CSS patient.
The two present cases showed high levels of serum MPO-ANCA titer. Although the pathogenic role of MPO-ANCA in CSS is not well understood, positive ANCA is thought to be involved in intracranial hemorrhage, such as organ system manifestations, including nephropathy, peripheral neuropathy, and purpura.
In review of previously described cases that presented cerebral hemorrhage in patients with CSS, in almost all patients, including our cases, cerebral hemorrhage occurred in phase of active disease. This is pointed out by the presence of hypereosinophilia and increased blood inflammatory markers. Also, in our cases, ECP, a marker of disease activity, was elevated. These observations could be a differential diagnostic point with other causes of cerebral hemorrhage, such as hypertensive ICH.
Cyclophosphamide combined with glucocorticoids is the standard therapy for remission induction in patients with CSS. These treatments are also indicated in patients with CNS involvement.16) In patients already diagnosed with CSS, if new neurological presentations develop, the possibility of ICH should be considered. Also, in patients with a history of asthma and allergic rhinitis, unknown causes of a stroke may be a complication of CSS. This is important because cerebral involvement is a major cause of morbidity and mortality.
We report on two cases of SAH and ICH, as a presentation of CSS. Despite being rare, SAH and ICH can be presenting features of vasculitis associated with CSS. In this regard, CSS vasculitis should be considered in the differential diagnosis of hemorrhagic stroke, because prompt management of ICH in CSS, which was performed immediately after diagnosis, can minimize morbidity and mortality.
Figures and Tables
Fig. 1
Brain three dimensional computed tomography (3D CT) angiography. Brain CT showed a thick subarachnoid hemorrhage, including in peri-medullary cistern (A). Brain CTA revealed a left-sided vertebral artery dissection (VAD) and aplasia on the contra-lateral side VA (B).
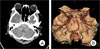
Fig. 2
Cerebral angiography. Digital subtraction angiography (DSA) image demonstrates an extravasation of the contrast media form dissected segment (A). Postembolization angiography demonstrates complete occlusion of the segment of the extravasation and the flow of the dominant left VA was preserved (B).

References
1. Calvo-Romero JM, del Carmen Bonilla-Gracia M, Bureo-Dacal P. Churg-Strauss Syndrome presenting as spontaneous subarachnoid haemorrhage. Clin Rheumatol. 2002. 06. 21(3):261–263.

2. Chang Y, Kargas SA, Goates JJ, Horoupian DS. Intraventricular and subarachnoid hemorrhage resulting from necrotizing vasculitis of the choroid plexus in a patient with Churg-Strauss syndrome. Clin Neuropathol. 1993. Mar-Apr. 12(2):84–87.
3. Churg J, Strauss L. Allergic granulomatosis, allergic angiitis, and periarteritis nodosa. Am J Pathol. 1951. Mar-Apr. 27(2):277–301.
4. Guillevin L, Cohen P, Gayraud M, Lhote F, Jarrousse B, Casassus P. Churg-Strauss syndrome. Clinical study and long-term follow-up of 96 patients. Medicine (Baltimore). 1999. 01. 78(1):26–37.

5. Halliday J, Herrick A, Leach J. Churg-Strauss, a rare cause of intracerebral haemorrhage. J Clin Neurosci. 2012. 08. 19(8):1177–1178.

6. Hattori N, Ichimura M, Nagamatsu M, Li M, Yamamoto K, Kumazawa K, et al. Clinicopathological features of Churg-Strauss syndrome-associated neuropathy. Brain. 1999. 03. 122(Pt 3):427–439.

7. Lanham JG, Elkon KB, Pusey CD, Hughes GR. Systemic vasculitis with asthma and eosinophilia: a clinical approach to the Churg-Strauss syndrome. Medicine (Baltimore). 1984. 03. 63(2):65–81.
8. Liou HH, Liu HM, Chiang IP, Yeh TS, Chen RC. Churg-Strauss syndrome presented as multiple intracerebral hemorrhage. Lupus. 1997. 6(3):279–282.

9. Masi AT, Hunder GG, Lie JT, Michel BA, Bloch DA, Arend WP, et al. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum. 1990. 08. 33(8):1094–1100.

10. Mencacci NE, Bersano A, Cinnante CM, Ciammola A, Corti S, Meroni PL, et al. Intracerebral haemorrhage, a possible presentation in Churg-Strauss syndrome: case report and review of the literature. J Neurol Sci. 2011. 02. 301(1-2):107–111.

11. Mishra S, Das CP, Das A, Prabhakar S. Intracerebral hemorrhage in a patient with Churg-Strauss syndrome. Neurol India. 2007. Oct-Dec. 55(4):416–418.

12. Nam TS, Jung HJ, Kim JT, Park MS, Kim BC, Kim MK, et al. [Churg-Strauss Syndrome Complicated With Intracerebral Hemorrhage]. J Korean Neurol Assoc. 2009. 08. 27(3):257–259. Korean.
13. Nishino R, Murata Y, Oiwa H, Arakawa T, Sunakawa M, Tsuge M, et al. [A case of Churg-Strauss syndrome presented as right thalamic hemorrhage]. No To Shinkei. 1999. 10. 51(10):891–894. Japanese.
15. Ojeda E, Auzmendi A, Teresa Unanue M, Fathi O. [Cerebral hemorrhage in Churg-Strauss syndrome]. Med Clin (Barc). 001. 01. 116(3):118–119. Spanish.
16. Ramakrishna G, Midthun DE. Churg-Strauss syndrome. Ann Allergy Asthma Immunol. 2001. 06. 86(6):603–613. quiz 13.

17. Sairanen T, Kanerva M, Valanne L, Lyytinen J, Pekkonen E. Churg-Strauss syndrome as an unusual aetiology of stroke with haemorrhagic transformation in a patient with no cardiovascular risk factors. Case Rep Neurol. 2011. 01. 3:32–38.

18. Sakamoto S, Ohba S, Eguchi K, Shibukawa M, Kiura Y, Okazaki T, et al. Churg-Strauss syndrome presenting with subarachnoid hemorrhage from ruptured dissecting aneurysm of the intracranial vertebral artery. Clin Neurol Neurosurg. 2005. 08. 107(5):428–431.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download