Abstract
The optimal treatment and appropriate follow-up period for an unruptured vertebral artery (VA) and/or posterior inferior cerebellar artery (PICA) dissection have not been established. Decisions regarding treatment of these vascular lesions are usually based on the manifesting symptoms and changes in radiologic findings during the follow-up period. We experienced a patient who had a simultaneous unruptured VA dissection and a contralateral PICA dissecting aneurysm. We did not find such a case in other literature.
Intracranial arterial dissections occur more often in posterior circulation, and can cause subarachnoid hemorrhage (SAH) and/or cerebral ischemia.18) Due to a high rebleeding rate and mortality, ruptured dissecting aneurysms require early treatment by endovascular obliteration or open surgery.5)14)15) However, understanding of the natural history and optimal management of spontaneous unruptured vertebral artery (VA) and/or posterior inferior cerebellar artery (PICA) dissection is incomplete.3)4)6)10) We experienced a patient who had a simultaneous unruptured VA dissection and a contralateral PICA dissecting aneurysm. Considering the bleeding risk at the acute stage, the unruptured VA dissection was treated via endovascular stent insertion. However, due to its technically low availability, the PICA lesion was conserved without endovascular treatment.
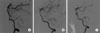
A 42-year-old previously healthy male patient complained of sudden onset of a severe right-sided occipital headache for one day. Although he had dizziness and nausea, findings on his neurological examination were normal. He had no history of trauma or remarkable medical or familial history. Findings on a computed tomographic (CT) scan performed on admission showed no evidence of intracranial hemorrhage. In addition, no abnormality was observed on magnetic resonance imaging (MRI). However, a high signal intensity representing an intramural hematoma was distinguished from a flow-related enhancement of the VA on the source image of time-of-flight (TOF) - magnetic resonance angiography (MRA) (Fig. 1A). The MRA showed tapered narrowing of the right VA at the junction of the right VA and PICA, and fusiform dilatation of the anterior medullary segment of the left PICA (Fig. 1B). On the lumbar tapping, there was no evidence of SAH. Cerebral angiography performed the next day showed a "pearl and string" sign and a double lumen sign in the right distal V4 segment of the VA involving the origin of the right PICA, indicating a spontaneous dissecting aneurysm (Fig. 2A), and focal stenosis with a post-stenotic fusiform aneurysmal dilatation of the left proximal PICA, suggesting a dissection with an aneurysm formation (Fig. 3A). Endovascular treatment of the right VA dissection was performed immediately after cerebral angiography: a Prowler Selector Plus microcatheter (Cordis Neurovascular, Miami, FL) was placed through the dissection segment in the right VA; then, two Enterprise stents (Cordis Neurovascular, Miami, FL) were deployed to the right VA dissection site in order to dilate it to cover the dissection (Fig. 2B). Several possible treatment options were considered for the suggestive dissecting aneurysm on the left proximal PICA. Finding clues of a dissection in this small vessel was difficult; thus, we could not rule out the likelihood of an originally existing fusiform aneurysm. In addition, the left PICA lesion was technically less available; the diameter of the stenotic segment was too narrow to perform a stent insertion. Therefore, we made the decision to provide conservative treatment. The patient's symptoms showed gradual improvement and he recovered without complications. The second angiography performed at four weeks after symptom onset confirmed that the right VA dissection lesion had not recurred and a reduction was observed in the left PICA dilatation (Fig. 3B). This finding suggested that the left PICA lesion was a dissecting aneurysm in a spontaneous healing state. Findings on follow-up vertebral arteriography three months later showed no recurrence of the right VA dissection lesion (Fig. 2C) and spontaneous resolution of the left PICA dilatation (Fig. 3C).
Dissecting aneurysms of the VA account for 1.4% of all intracranial aneurysms and 11% of the aneurysms located in posterior circulation.15) PICA aneurysms account for approximately 0.5% to 3.0% of all intracranial aneurysms.5) Knowledge of the incidence of simultaneous dissection of the VA and the contralateral PICA is limited, and the cause or pathogenesis of multiple intracranial dissections is unclear.
The most common clinical feature of an unruptured VA dissection is a severe occipital headache only or focal ischemia of posterior circulation. The initial presentation of patients is critical to the prognosis of such lesions;4)6) however, their optimal management and appropriate length of the follow-up period have not been established. Some studies have reported a relatively good prognosis for patients with an unruptured VA dissection.14)16)19) However, Miszutani et al.11) reported occurrence of hemorrhage within three days after ictus in two thirds of unruptured VA dissections. These findings suggest that if the first few days pass without hemorrhage, the clinical outcome for patients with unruptured VA dissections may be good. Naito et al.13) found that the hemorrhage from unruptured VA dissections was more often than previously considered. In their evaluation of 21 patients with unruptured VA dissections, three patients subsequently experienced hemorrhage; in two patients, hemorrhage occurred within one day after the preceding headache. They recommend endovascular treatment for unruptured VA dissections associated with relatively large aneurysmal dilatations or a double-lumen sign in the acute stage and with growing aneurysmal dilatations during the follow-up period.
In our case, intramural hematoma was detected in the source image of a TOF-MRA, and the pearl and string and double lumen signs were shown as a characteristic MRA and conventional angiography findings of VA dissections. Because an increased risk of rupture has been demonstrated for VA dissections showing these angiographic findings in the acute stage, treatment such as endovascular or direct surgery is required.13) Thus, in this case, endovascular treatment of the right VA dissection was performed immediately after cerebral angiography. In particular, with the advance of endovascular techniques, use of stents and coils may be one of the best treatment strategies for prevention of rupture of an unruptured VA dissection. Some patients whose VA dissections were treated by stenting, with or without coiling, have had favorable clinical outcomes.6)7) The aim of this management using stents and coils is the preservation of the parent artery flow. In our case, because of a VA dissection involving the PICA origin, patency of the VA and PICA should be preserved with stent placement. In addition, the risk of rupture of a VA dissection is higher in the acute stage. Therefore, we posited that stenting would be a suitable treatment for right VA dissection. Serial angiographic follow-up studies indicated that the patency of the VA was maintained after stenting.
Previous studies of dissecting PICA aneurysms have reported that in spontaneous PICA dissection, treatment with careful observation may be preferable to surgical or endovascular intervention, if there are no obvious angiographic risk factors for hemorrhage, such as a pseudoaneurysm.3)14)18) In our case, although we suggested that this fusiform lesion represents arterial dissection, finding the angiographic feature of dissection in this small vessel was difficult. Endovascular treatment of a PICA lesion was likely to be less available technically. In addition, any treatment, including occlusion of a proximal PICA may lead to infarction of the medulla oblongata, due to the perforators supplying this structure.1)2) Hence, instead of using aggressive treatment, we treated the left PICA dissecting aneurysm conservatively. We decided to perform a short-term follow-up vertebral arteriography. Serial vertebral arteriography performed during the three-month follow-up indicated spontaneous improvement and gradual healing of the dissection site on the left PICA.
The most important mechanism of arterial dissection is the disruption of the internal elastic lamina.12) The healing process for dissecting aneurysms may be based on formation of the intima. In the subsequent healing process, T cells and macrophages are activated on the first day, endothelium covers the lesion within three to five days, synthetic smooth muscle cells begin to form within four days, and the neointima appears after one week. At three months, intimal formation is complete.17) Therefore, neointima formation, reinforcing the dissection site, is completed between one week and three months.11) The appropriate length of follow-up in patients with unruptured VA dissections has not been established. In some cases, changes at the dissection site were observed two to three months post onset,8)19) whereas in others, an MRI performed six months after initial symptoms indicated the disappearance of the high signal mural hematoma at the dissection site.7)9) Kai et al.6) have suggested that patients with unruptured VA dissections should be monitored closely for six months, and that an active follow-up period of more than two years is not required. In our case, follow-up vertebral angiography performed at three months showed a good patency of the right VA and spontaneous resolution of the left PICA dilatation. We suggest that patients who have VA dissections and/or PICA dissections need at least three months follow-up angiography after symptom onset.
We report on a rare case of simultaneous unruptured VA dissection and contralateral PICA dissection. Treatment should be considered for symptomatic unruptured VA dissections in the acute stage. In dissecting PICA aneurysms, especially non-hemorrhagic lesions, there is a possibility of spontaneous resolution resulting in a favorable outcome. Decisions regarding treatment for these vascular lesions may be based on the manifesting symptoms and radiologic changes observed on careful follow-up.
Figures and Tables
Fig. 1
On source image of time-of-flight (TOF) - magnetic resonance angiography (MRA), high signal intensity (arrow) representing an intramural hematoma is distinguished from flow-related enhancement of the VA (A). The MRA shows a faint signal adjacent to the junction of the right VA and PICA (arrowhead) and a fusiform dilatation of the anterior medullary segment of the left PICA (arrow) (B).
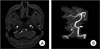
Fig. 2
A right vertebral angiogram shows the "pearl and string" sign and appearance of a double lumen in the right distal V4 segment of the vertebral artery (VA) involving the origin of the right posterior inferior cerebellar artery (PICA) (A). The follow-up angiograms immediately and three months after stent placement demonstrate good patency of the VA (B, C).
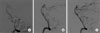
Fig. 3
A left vertebral angiogram shows focal stenosis with a post-stenotic fusiform aneurysmal dilatation (arrow) of the left proximal posterior inferior cerebellar artery (PICA) suggesting dissection with pseudoaneurysm formation (A). The second angiography at four weeks after symptom onset confirms the reduction of the left PICA dilatation (arrow) (B). Follow-up vertebral arteriography three months later indicates spontaneous resolution of the left PICA dilatation (arrow) (C).
References
1. Ali MJ, Bendok BR, Tawk RG, Getch CC, Batjer HH. Trapping and revascularization for a dissecting aneurysm of the proximal posteroinferior cerebellar artery: technical case report and review of the literature. Neurosurgery. 2002. 07. 51(1):258–262. discussion 262-3.

2. Bradac GB, Bergui M. Endovascular treatment of the posterior inferior cerebellar artery aneurysms. Neuroradiology. 2004. 12. 46(12):1006–1011.

3. Fransen P, de Tribolet N. Dissecting aneurysm of the posterior inferior cerebellar artery. Br J Neurosurg. 1994. 8(3):381–386.

4. Friedman AH, Drake CG. Subarachnoid hemorrhage from intracranial dissecting aneurysm. J Neurosurg. 1984. 02. 60(2):325–334.

5. Isokangas JM, Siniluoto T, Tikkakoski T, Kumpulainen T. Endovascular treatment of peripheral aneurysms of the posterior inferior cerebellar artery. AJNR Am J Neuroradiol. 2008. 10. 29(9):1783–1788.

6. Kai Y, Nishi T, Watanabe M, Morioka M, Hirano T, Yano S, et al. Strategy for treating unruptured vertebral artery dissecting aneurysms. Neurosurgery. 2011. 11. 69(5):1085–1091. discussion 1091-2.

7. Kasner SE, Hankins LL, Bratina P, Morgenstern LB. Magnetic resonance angiography demonstrates vascular healing of carotid and vertebral artery dissections. Stroke. 1997. 10. 28(10):1993–1997.

8. Kitanaka C, Tanaka J, Kuwahara M, Teraoka A, Sasaki T, Takakura K, et al. Nonsurgical treatment of unruptured intracranial vertebral artery dissection with serial follow-up angiography. J Neurosurg. 1994. 04. 80(4):667–674.

9. Leclerc X, Lucas C, Godefroy P, Nicol L, Moretti A, Leys D, et al. Preliminary experience using contrast-enhanced MR angiography to assess vertebral artery structure for the follow-up of suspected dissection. AJNR Am J Neuroradiol. 1999. 09. 20(8):1482–1490.
10. Lv X, Jiang C, Li T, Wu Z. Clinical outcomes of ruptured and unruptured vertebral artery-posterior inferior cerebellar artery complex dissecting aneurysms after endovascular embolization. AJNR Am J Neuroradiol. 2010. 08. 31(7):1232–1235.

11. Mizutani T, Kojima H, Asamoto S. Healing process for cerebral dissecting aneurysms presenting with subarachnoid hemorrhage. Neurosurgery. 2004. 02. 54(2):342–347. discussion 347-8.

12. Mizutani T, Kojima H, Miki Y. Arterial dissections of penetrating cerebral arteries causing hypertension-induced cerebral hemorrhage. J Neurosurg. 2000. 11. 93(5):859–862.

13. Naito I, Iwai T, Sasaki T. Management of intracranial vertebral artery dissections initially presenting without subarachnoid hemorrhage. Neurosurgery. 2002. 10. 51(4):930–937. discussion 937-8.

14. Nakagawa K, Touho H, Morisako T, Osaka Y, Tatsuzawa K, Nakae H, et al. Long-term follow-up study of unruptured vertebral artery dissection: clinical outcomes and serial angiographic findings. J Neurosurg. 2000. 07. 93(1):19–25.

15. Niijima K. Dissecting aneurysm of the vertebral artery with an accessory posterior inferior cerebellar artery: successful management with clipping between the two posterior inferior cerebellar arteries. Cerebrovasc Dis. 2001. 11(2):138–140.

16. Santos-Franco JA, Zenteno M, Lee A. Dissecting aneurysms of the vertebrobasilar system. A comprehensive review on natural history and treatment options. Neurosurg Rev. 2008. 04. 31(2):131–140. discussion 140.

17. Stary HC, Chandler AB, Glagov S, Guyton JR, Insull W Jr, Rosenfeld ME, et al. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1994. 05. 89(5):2462–2478.

18. Tawk RG, Bendok BR, Qureshi AI, Getch CC, Srinivasan J, Alberts M, et al. Isolated dissections and dissecting aneurysms of the posterior inferior cerebellar artery: topic and literature review. Neurosurg Rev. 2003. 07. 26(3):180–187.
19. Yoshimoto Y, Wakai S. Unruptured intracranial vertebral artery dissection. Clinical course and serial radiographic imagings. Stroke. 1997. 02. 28(2):370–374.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download