Abstract
Introduction
Patients with negative initial digital subtraction angiography (DSA) are at significant risk for re-bleeding, which can lead to severe disability and death. The purpose of this study was to evaluate the necessity of repeat DSA in subgroups of patients with subarachnoid hemorrhage (SAH) with negative initial DSA.
Methods
A total of 904 spontaneous SAH patients were admitted to our department between May 2005 and May 2012. Twenty eight patients were selected for inclusion in this study because repeated DSA performed due to the etiology of the SAH could not be demonstrated on the initial DSA. According to the SAH pattern on initial computed tomography scans, patients were divided into perimesencephalic nonaneurysmal SAH (PN-SAH) and non PN-SAH (NPN-SAH) groups. Repeat DSA was performed in all patients, and two of these patients underwent a third DSA.
Results
Of the 904 patients, 28 patients (3.1%) had no vascular abnormality on initial DSA. Sixteen PN-SAH patients underwent a repeat DSA; however, no aneurysms were found. In contrast, 12 patients with NPN-SAH underwent repeat DSA, with detection of two cerebral aneurysms. Overall, the false-negative rate of the initial DSA was 7.1% (2/28 patients). No significant differences in false-negative results on initial DSA were observed between the PN-SAH and NPN-SAH groups.
Conclusion
In the line with the results of the current study, we should be highly suspicious of patients with a nonaneurysmal SAH, especially those with a NPN-SAH pattern. In order to reduce the morbidity and mortality resulting from a misdiagnosis, repeat DSA is necessary, and exclusion of an aneurysm is important.
The incidence of initial digital subtraction angiography (DSA) negative subarachnoid hemorrhage (SAH) is approximately 15% of all patients.4) Perimesencephalic nonaneurysmal SAH (PN-SAH) was first described by Van Gijn et al.13) in 1985 as a computed tomography (CT) scan pattern of hemorrhage restricted to the perimesencephalic or prepontine cisterns, no aneurysmal structure in DSA, and low rates of re-bleeding and vasospasm with good recovery.8)9) However, in patients with non-diagnostic initial DSA, a significant risk for re-bleeding persists (e.g, from vascular malformation or undetected aneurysm), which can result in considerable morbidity and death.9) It is important to determine whether a cerebral aneurysm exists but cannot be found, or whether the hemorrhage is the result of other unknown causes. For this reason, repeat DSA has been performed after a negative initial DSA. False-negative rates have shown a decrease following improvements in diagnostic imaging capabilities, however, the need for repeat DSA in spontaneous SAH patients with a negative initial DSA is still being debated. While DSA has high sensitivity and specificity for detection of cerebral aneurysms, this invasive procedure is associated with some complications (SAH-specific mortality in 0.17% of patients, neurological morbidity in 3.2% of patients, with permanent disability in 0.04%).5) Given the variety of available diagnostic tests and differences in their associated risks and costs, it is important to determine the diagnostic yield of each test and to compare the yield between SAH subgroups in which initial DSA was negative.11) The purpose of this study was to evaluate the necessity of repeating DSA in SAH patients with negative initial DSA.
A total of 904 spontaneous SAH patients were admitted to our department of neurological surgery between May 2005 and May 2012. DSA was performed for initial evaluation on 458 patients. Criteria for patients of this study included the following: SAH was easily confirmed by initial CT scan, and patients with an SAH etiology that could not be verified on initial DSA, and on whom at least one more repeated DSA was performed. A monoplane neuro X-ray system (Allura Xper FD20; Philips, Best, the Netherlands) was used in performance of DSA examinations. Of the 28 patients who underwent a repeat DSA, two of these patients underwent even a third DSA. According to the SAH pattern on initial CT scans, patients were divided into two groups. PN-SAH comprises (1) a center hemorrhage located immediately in front of the midbrain or within perimesencephalic, prepontine, or medullary cisterns; (2) absence of intraparenchymal bleeding; (3) extension of blood into the sylvian fissure with no more than a minute amount of blood in the lateral sylvian fissure; (4) extension of blood without complete filling of the anterior interhemispheric fissure; (5) absence of a frank intraventricular hemorrhage (sedimentation of a small amount of intraventricular blood is allowed).10) Hemorrhages that did not meet all of these criteria were classified as non PN-SAH (NPN-SAH). None of the patients was diagnosed with SAH by lumbar puncture after a negative CT scan. All patients underwent CT angiography, which was negative for structural pathology and subsequently underwent DSA. Clinical grade was based on the Glasgow Coma Scale (GCS) and Hunt-Hess (H-H) grading system at the time of admission. Functional outcome was assessed according to the Glasgow Outcome Scale (GOS). All patients received conservative treatment according to the standard SAH protocols, which included adequate hydration, calcium channel blockers (Nimodipine), and anticonvulsants during the acute stage. The SPSS program (version 12.0; SPSS Inc., Chicago, IL) was used in performance of chi-square tests and t-tests.
Of the 904 patients with spontaneous SAH, 28 patients (3.1%) had no vascular abnormality on initial DSA. Repeated DSA was performed within 5-13 (mean 8.2) days for patients in whom the initial DSA did not reveal any aneurysmal structures or vascular malformation. Fourteen male and 14 female patients (M:F = 1:1) were included and the mean age was 60.3 (range 37-77). Initial CT scans showed PN-SAH patterns in 16 patients (57.1%) and NPN-SAH patterns in 12 patients (42.9%). Patients' demographic data were presented with their clinical grades on admission (Table 1). The PN-SAH group had relatively lower Fisher grade distributions when compared to the NPN-SAH group (p < 0.001). The GOS was used for analysis of clinical outcomes. Every patient in the PN-SAH group and 75% of patients in the NPN-SAH group showed recovery to grade 5 (Table 2). Regardless of their initial SAH patterns, most of the patients had good clinical outcomes upon discharge. No statistically significant difference was observed between the PN-SAH group and NPN-SAH group (p = 0.096). Sixteen PN-SAH patients underwent a repeat DSA, however, no aneurysm was found. In contrast, 12 NPN-SAH patients underwent repeat DSA, with detection of cerebral aneurysms in two patients. One patient had re-bleeding of the supraclinoid dorsal wall aneurysm, and a supraclinoid dorsal wall aneurysm was detected in the other patient following a repeated DSA. The overall false-negative rate of the initial DSA was 7.1% (2/28 patients).
A 67-year-old female patient complaining of headache, dizziness, and nausea visited our emergency department. Neurologic examination revealed no focal neurological deficits, except for neck stiffness. Findings on brain noncontrast CT and CT angiography revealed moderate SAH, predominantly in the right sylvian fissure, however, no aneurysm was detected. DSA was performed, but it did not show any aneurismal structure or vascular malformation. The patient received conservative treatment in the intensive care unit and recovered without any complications. Repeat DSA on the eighth day after SAH revealed a dorsal wall aneurysm measuring 3.0 × 1.3 mm on the right distal internal carotid artery (ICA) (Fig. 1).
A 37-year-old male patient was transferred to our hospital for suspicion of SAH with headache, left side motor weakness, and dysarthria. Neurologically, the patient had a drowsy consciousness level and grade III left-side motor weakness. Findings on CT scan revealed scanty hemorrhage on the right sylvian fissure and CT angiography revealed an occlusion of the right M1 segment of the middle cerebral artery, however, no aneurysmal lesions were found. We performed brain magnetic resonance imaging (MRI), which revealed an acute cerebral infarction on the right striatocapsular area. DSA was performed on the day of hemorrhage, however, angiograms did not show any aneurysmal structure, except for the right middle cerebral artery occlusion (Fig 2). Because the patient's leptomeningeal collateral circulation was sufficient, we did not perform a mechanical thrombolysis. The patient received treatment in accordance with standard clinical practice for acute cerebral infarction in the intensive care unit and recovered grade IV left-side motor weakness. On the sixth day of admission, he exhibited deterioration of his consciousness level, with a tonic-clonic seizure. CT showed a large amount of SAH at the basal cistern, an interhemispheric fissure, and both sylvian fissures due to re-bleeding. We immediately performed a DSA, which revealed a dorsal wall aneurysm measuring 4.7 × 2.3 mm on the right ICA ophthalmic segment (Fig. 3).
The main cause of spontaneous SAH was aneurysmal rupture, and misdiagnosis results in severe neurologic disability and death. DSA is good modality for aneurysm detection. However, in 15% of patients with spontaneous SAH, no aneurysmal structure is found on the initial DSA.4) This might be due to a very small microaneurysm, occult aneurysm concealed by hemorrhage or vasospasm, hemorrhage from a venous system, or inadequate technique.2)6) Although these misdiagnose may have been unavoidable, the result is severe neurologic disability and death. Therefore, when initial DSA revealed no aneurysm, repeat DSA is required in order to reduce the incidence of morbidity and mortality due to misdiagnosis.
In the current study, all patients who had negative findings on the initial DSA underwent repeat DSA; 28 of the patients had a negative initial DSA, and cerebral aneurysms were found in two patients (false-negative rate 7.1%). The rate of misdiagnosis was reduced, compared to other studies, which might be due to developments in the capacity of diagnostic DSA. We evaluated the necessity of repeat DSA with negative initial DSA according to the SAH pattern. PN-SAH is a distinct characteristic imaging pattern of nontraumatic, nonaneurysmal SAH, which is associated with a good clinical outcomes.13) Compared to aneurysmal SAH, it usually presents only with headaches and no mental deterioration and with a good clinical grade.9)13) On admission, clinical grades corresponding to GCS scores and the GOS score at discharge was higher in the PN-SAH group than in the NPN-SAH group; however, these results showed no statistical significance. Most of these patients had good clinical outcomes, however, one patient in the NPN-SAH group died due to re-bleeding and consequential vasospasm.
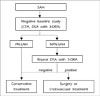
Two patients had an aneurysm on repeat DSA and the size and shape of the aneurysms were changed between the initial and repeat DSA. These two aneurysms were located on the dorsal wall of the ICA. Two of the false-negative initial DSA patients showed an NPN-SAH pattern on the initial CT scan. When compared to the PN-SAH group, the NPN-SAH group had a high false-negative rate (16.7%) (0%, p = 0.175). Based on the results of our study, because structural abnormalities, such as aneurysms, can be obscured in the first angiogram, repeated DSA is always indicated in patients with NPN-SAH patterns upon CT scan and negative initial DSA. In particular, configurational changes have been observed in serial cerebral angiography of supraclinoid dorsal wall aneurysms. Repeated DSA should result in reduced false-negative rates and the incidence of re-bleeding. PN-SAH patterns have a very high predictive value for normal angiogram.10) Due to improvements in diagnostic imaging capabilities, such as three-dimensional rotational angiography (3-DRA), the recent incidence of DSA-negative SAH has shown a remarkable reduction. This technique is better for resolving complicated anatomy, allowing the investigator to detect aneurysms that are otherwise not visible when using conventional DSA. Ishihara et al.4) reported that the incidence of DSA-negative SAH was 8.6% in the DSA group and 4.2% in the 3-DRA group. Although DSA with 3-DRA is the gold standard for detection of aneurysms, it is invasive and may be associated with neurological complications. In our series of 16 PN-SAH patients, the cause of bleeding was not detected through repeated DSA. Thus, provided that the initial DSA was technically adequate and revealed no vasospasm, a repeat DSA might not be required.11) We advocate the need for only a single DSA with 3-DRA in the PN-SAH group. Due to the benign clinical course of this subgroup, we believe that the risks of DSA are too high in comparison with CT angiography.12)14) CT angiography has multiple advantages over DSA, as it is a noninvasive, widely available technique, which requires shorter time, and uses less contrast media than the DSA performed in patients with negative CT angiography.3) However, when the hemorrhage shows an NPN-SAH pattern, repeat DSA is necessary due to the possibility of aneurysmal SAH, even if the initial DSA is negative (Fig. 4). Configurational changes have occasionally been observed in repeat DSA: blister-like aneurysms have shown changes in configuration into a saccular type and have even shown spontaneous regression.1)7)
In the current study, compared to previous studies, the rate of misdiagnosis was reduced, owing to the development of diagnostic DSA techniques, including 3-DRA. However, all clinicians should be highly suspicious of patients with non-aneurysmal SAH, especially those with an NPN-SAH pattern, and 3-DRA should be performed during the initial DSA for patients with SAH of unknown etiology upon the initial CT scan and CT angiogram. It is necessary for patients in the NPN-SAH group to undergo repeat DSA in combination with 3-DRA in order to exclude aneurysms, which is important for reducing morbidity and mortality due to misdiagnosis.
Figures and Tables
Fig. 1
Initial noncontrast brain computed tomography (CT) and digital subtraction angiography (DSA). Diffuse subarachnoid hemorrhage (SAH) in the region of both the sylvian fissure and interhemispheric fissure (A). Initial three-dimensional rotational angiography image reveals no aneurysmal structure (B). Repeat DSA was performed on the eighth day of admission. Three-dimensional rotational angiography images clearly show a distal ICA aneurysm (C, D).

Fig. 2
Initial brain CT, magnetic resonance imaging (MRI) and DSA on admission. Brain noncontrast CT and MRI flair images show a subarachnoid hemorrhage on the right sylvian fissure (A, B), Mid-arterial phase DSA images show occlusion of the right M1 segment of the middle cerebral artery, but reveal no aneurysmal structure (C, D).

Fig. 3
Brain CT and repeat DSA on the sixth day of admission. A large amount of SAH is observed in the region of the basal cistern, both sylvian fissures, and interhemispheric fissure (A). Repeat DSA and three-dimensional angiography show an aneurysm on the ophthalmic segment of the right internal cerebral artery, probably a blister-like dorsal wall aneurysm (B, C, D).

References
1. Abe M, Tabuchi K, Yokoyama H, Uchino A. Blood blisterlike aneurysms of the internal carotid artery. J Neurosurg. 1998. 09. 89(3):419–424.

2. Broderick JP, Brott TG, Duldner JE, Tomsick T, Leach A. Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke. 1994. 07. 25(7):1342–1347.

3. Cruz JP, Sarma D, Noel de Tilly L. Perimesencephalic subarachnoid hemorrhage: when to stop imaging? Emerg Radiol. 2011. 06. 18(3):197–202.

4. Ishihara H, Kato S, Akimura T, Suehiro E, Oku T, Suzuki M. Angiogram-negative subarachnoid hemorrhage in the era of three dimensional rotational angiography. J Clin Neurosci. 2007. 03. 14(3):252–255.

5. Kaufmann TJ, Huston J 3rd, Mandrekar JN, Schleck CD, Thielen KR, Kallmes DF. Complications of diagnostic cerebral angiography: evaluation of 19,826 consecutive patients. Radiology. 2007. 06. 243(3):812–819.
6. McMahon J, Dorsch N. Subarachnoid haemorrhage of unknown aetiology: what next? Crit Rev Neurosurg. 1999. 05. 9(3):147–155.

7. Ogawa A, Suzuki M, Ogasawara K. Aneurysms at nonbranching sites in the supraclinoid portion of the internal carotid artery: internal carotid artery trunk aneurysms. Neurosurgery. 2000. 09. 47(3):578–583. discussion 83-6.

8. Rinkel GJ, Wijdicks EF, Vermeulen M, Hageman LM, Tans JT, van Gijn J. Outcome in perimesencephalic (nonaneurysmal) supraclinoid hemorrhage: a follow-up study in 37 patients. Neurology. 1990. 07. 40(7):1130–1132.
9. Rinkel GJ, Wijdicks EF, Hasan D, Kienstra GE, Franke CL, Hageman LM, et al. Outcome in patients with subarachnoid haemorrhage and negative angiography according to pattern of haemorrhage on computed tomography. Lancet. 1991. 10. 338(8773):964–968.

10. Rinkel GJ, Wijdicks EF, Vermeulen M, Ramos LM, Tanghe HL, Hasan D, et al. Nonaneurysmal perimesencephalic subarachnoid hemorrhage: CT and MR patterns that differ from aneurysmal rupture. AJNR Am J Neuroradiol. 1991. Sep-Oct. 12(5):829–834.
11. Topcuoglu MA, Ogilvy CS, Carter BS, Buonanno FS, Koroshetz WJ, Singhal AB. Subarachnoid hemorrhage without evident cause on initial angiography studies: diagnostic yield of subsequent angiography and other neuroimaging tests. J Neurosurg. 2003. 06. 98(6):1235–1240.

12. van Gijn J, Rinkel GJ. Subarachnoid haemorrhage: diagnosis, causes and management. Brain. 2001. 02. 124(Pt 2):249–278.

13. van Gijn J, van Dongen KJ, Vermeulen M, Hijdra A. Perimesencephalic hemorrhage: a nonaneurysmal and benign form of subarachnoid hemorrhage. Neurology. 1985. 04. 35(4):493–497.

14. Velthuis BK, Rinkel GJ, Ramos LM, Witkamp TD, van Leeuwen MS. Perimesencephalic hemorrhage. Exclusion of vertebrobasilar aneurysms with CT angiography. Stroke. 1999. 05. 30(5):1103–1109.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download