Abstract
Objective
A dural arteriovenous fistula (DAVF) generally refers to a vascular malformation of the wall of a major venous sinus. These lesions have diverse symptoms according to the location and venous drainage, and require multidisciplinary treatment. We report on our experience and analyze the treatment outcome of intracranial DAVFs for a nine-year period.
Methods
Between January 2000 and December 2008, 95 patients with intracranial DAVFs were enrolled in this study. A retrospective review of clinical records and imaging studies of all patients was conducted. Endovascular embolization, surgical interruption, gamma knife stereotactic radiosurgery (GKS), or combinations of these treatments were performed based on clinical symptoms, lesion location, and venous drainage pattern.
Results
Borden type I, II, and III were 34, 48, and 13 patients, respectively. Aggressive presentation was reported in 6% of Borden type I, 31% of Borden type II, and 77% of Borden type III DAVFs, respectively, and DAVFs involving transverse, sigmoid, and superior sagittal sinus. Overall, the rate of complete obliteration was 68%. The complete occlusion rates with a combination treatment of endovascular embolization and surgery, surgery alone, and endovascular embolization were 89%, 86%, and 80%, respectively. When GKS was used with embolization, the obliteration rate was 83%, although it was only 54% in GKS alone. Spontaneous obliteration of the DAVF occurred in three patients. There were a few complications, including hemiparesis (in microsurgery), intracranial hemorrhage (in endovascular embolization), and facial palsy (in GKS).
Conclusion
The hemorrhagic risk of DAVFs is dependent on the location and hemodynamics of the lesions. Strategies for treatment of intracranial DAVFs should be decided according to the characteristic of the DAVFs, based on the location and drainage pattern. GKS can be used as an optional treatment for intracranial DAVFs.
Dural arteriovenous fistulas (DAVFs) are pathologic shunts that develop between the dural arteries and dural venous sinuses, meningeal veins, or cortical veins. They account for approximately 10% to 15% of intracranial vascular malformations.11)14)19) They were once considered congenital, benign lesions, until Castaigne and Djindjian5) proposed an acquired etiology in the late 1970's. However, the benign nature of these lesions was challenged principally by Cognard et al.8) and Borden et al.,3) who proposed that the clinical aggressiveness of DAVFs was dependent on the degree of cortical venous reflux.
Intracranial DAVFs can be classified according to the type of venous drainage. The classification scheme developed by Cognard et al.8) and Borden et al.3) is the one most commonly used (Table 1). Using the Borden classification, lesions are categorized on the basis of the site of venous drainage, number of fistula (single-hole (a) or multiple-hole (b) fistulas), and the presence of cortical venous reflux (CVR). In the Cognard classification lesions are categorized according to the direction of dural sinus drainage, the presence of CVR, and the venous outflow architecture (nonectatic cortical veins, ectatic cortical veins, or spinal perimedullary veins).
Although the hemorrhagic risk of Borden type I or Cognard types I, IIa is extremely low, patients with high-grade DAVFs (Borden type II, III or Cognard IIb-V) have been documented as having increased risk for development of aggressive symptoms,3)8) such as intracranial hemorrhage, non-hemorrhagic neurologic deficits (NHNDs); seizures, Parkinsonism, cerebellar symptoms, apathy, failure to thrive, and cranial nerve deficits.17)26)28)
General therapeutic methods for management of DAVFs include conservative treatment, endovascular intervention, surgical treatment, and radiation therapy. Endovascular interventions have surged into the mainstream of DAVF therapy.11)
In this study, we analyze clinical characteristics of intracranial DAVFs with respect to hemorrhage and discuss current strategies for treatment of DAVFs.
Between January 2000 and December 2008, 95 patients with intracranial DAVFs, excluding carotid cavernous fistulas of traumatic origin, were enrolled in this study. These patients included 44 males and 51 females, with a mean age of 59.2 years, ranging from 16 to 82 years. We reviewed hospital records and imaging studies, including conventional angiography, during a follow-up period of 24.1 months, ranging from one to 89 months.
Conventional angiography, including injection of external carotid arteries for diagnosis and classification, was performed for evaluation of all patients with intracranial DAVFs. In most cases, computed tomographic angiography (CTA), magnetic resonance imaging (MRI), magnetic resonance angiography, and perfusion scanning were performed. Assessment of the patient's clinical presentation, current physical status, including comorbidities, and the radiological characteristics of the lesion was performed before embarking on any treatment.
These 95 patients were classified into three groups based on the Borden classification and categorized into six groups according to anatomic location: transverse-sigmoid sinus, cavernous sinus, superior sagittal sinus (SSS), tentorial, anterior cranial fossa, and others (jugular bulb, marginal sinus, deep venous fistula, torcular, brain stem, and other locations).
Aggressive clinical symptoms included intracranial hemorrhage and NHND. Both angiographic and clinical assessment of treatment outcomes for patients with DAVFs was performed. Angiographic outcomes were classified as incomplete or complete occlusion, based on the presence of a remaining arteriovenous shunt after treatment. The obliteration was considered complete when angiography showed no evidence of fistula. The Glasgow Outcome Scale (GOS) was used to assess the clinical outcomes after treatment.
Statistical analyses was performed using SPSS Statistics 20.0 (IBM, Chicago, IL). The Chi-square test and Fisher exact test were used selectively in performance of all statistical analyses. Statistical significance was set at a probability value (p value) less than 0.05.
Endovascular embolization, surgical interruption, Gamma Knife stereotactic radiosurgery (GKS), or a combination of these treatments was performed according to the clinical symptoms, lesion location, and venous drainage pattern. Conservative treatment was generally indicated in patients with DAVFs located in a cavernous sinus or with low-grade fistulas (Borden type I).
For most patients with DAVFs, endovascular treatment was generally recommended as the first treatment option for complete occlusion. Embolization was usually performed under general anesthesia. Endovascular treatment options included transarterial embolization, transvenous embolization, or a combination of both approaches using embolic agents, such as polyvinyl alcohol particles, n-butyl-2-cyanoacrylate, Onyx, or coils.
Surgery was recommended in the anterior cranial fossa DAVF and other complex lesions with drainage to SSS, transverse-sigmoid sinus. Due to the potential for massive bleeding, adequate craniotomy or craniectomy was performed for control of bleeding and to obliterate feeding vessels on the involved sinus and the surrounding dura under a navigation system. Preoperative angiography and intraoperative angiography were used for determination of whether or not the involved sinus was patent. If the involved sinus was patent, we preserved it and disconnected the arterialized veins from it and coagulated the surrounding dura.
GKS was applied independently in the lesions without cortical venous reflux, with a relatively low risk of intracranial hemorrhage, and with difficulties in performance of endovascular or surgical approaches. Dose planning was performed, paying attention to avoid excessive radiation exposure to radiosensitive structures. The mean volume was 6.9 cm3, ranging from 0.35 to 37.5 cm3. The mean margin dose was 19 Gy, ranging from 15 to 25 Gy, and the mean maximal dose was 38 Gy, ranging from 22 to 50 Gy, respectively.
When angiographically complete occlusion could not be obtained using single treatment modalities, a combination treatment was performed. If incomplete obliteration was observed, patients were followed by angiography for six to 12 months.
A majority of patients with DAVFs presented with non-aggressive symptoms. Thirty six patients (38%) had pulsatile tinnitus and bruit, 15 patients (16%) had ocular and visual symptoms, 12 patients (13%) had mild to moderate headache, and five patients (5%) were found incidentally.
Twenty seven patients (28%) presented with aggressive symptoms. Fifteen patients (16%) had intracranial hemorrhage, nine patients (9%) had seizure, and three patients (3%) had dementia, trigeminal neuralgia, and aphasia.
Four patients (31%) with DAVFs located in the SSS experienced seizure episodes. Intracranial hemorrhage was observed in eight patients (25%) with transverse-sigmoid sinus DAVF (Fig. 1), four patients (31%) with SSS DAVF, two patients (100%) with drainage of the DAVF into the torcular, and one patient (25%) with drainage into the vein of Galen, respectively. The majority of hemorrhages occurred in the transverse-sigmoid sinus and SSS DAVFs, however, intracranial hemorrhage with respect to the lesion location was not significant (p = 0.079, p = 0.211). No hemorrhage was observed in cavernous sinus DAVFs. A summary of the clinical symptoms and anatomic locations of the DAVFs is shown in Table 2.
The risk of hemorrhage and aggressive symptoms in DAVF with CVR (Borden type II and III) was 23% and 41%. More significant development of hemorrhage and aggressive symptoms was observed in DAVFs with CVR (Borden type II and III) (p = 0.009, p < 0.001) (Table 3).
Borden type II was the most common type, and the next most common was type I. Thirty four patients (36%) had Borden type I, 48 patients (50%) had Borden type II, and 13 patients (14%) had Borden type III.
According to the location of DAVFs, transverse-sigmoid sinus DAVFs were the most common. Thirty two patients (34%) had transverse-sigmoid sinus DAVFs, 15 patients (16%) had cavernous sinus DAVFs, 13 patients (13.5%) had SSS DAVFs, five patients (5%) had tentorial DAVFs, two patients (2%) had anterior cranial fossa DAVFs, and 28 patients (29%) had other locations. Cortical venous reflux (Borden type II and III) was more abundant in cavernous sinus DAVFs (94%) (p = 0.009). A summary of anatomic location and type according to the Borden classification is shown in Table 4.
Eighty six patients (91%) underwent surgical treatment, endovascular intervention, radiosurgery, or a combination treatment. Conservative treatment, including manual compression, was performed in nine patients (9%) with a cavernous sinus DAVF. Among them, spontaneously complete obliteration of cavernous sinus DAVFs was observed in three patients within 2, 22, and 29 months, respectively. Six out of nine patients who had undergone conservative treatment did not show complete obliteration and exhibited recurrent or newly developed symptoms.
Fifty nine patients (62%) were treated with endovascular therapy. Twenty of these patients were treated with embolization alone. Thirty nine patients who had shown incomplete obliteration with a single treatment modality were treated with combined therapy. Among them, 30 patients underwent a combined therapy of embolization and GKS, and, in the remaining nine patients, embolization and surgery were applied.
Complete occlusion determined by angiography was achieved in 25 patients (83%) treated with embolization and GKS during a mean follow-up period of 22 months (Fig. 1). Complete occlusion was achieved in eight patients (89%) treated with embolization and surgery during a mean follow-up period of 12 months. Sixteen patients (80%) who were treated with embolization alone showed complete occlusion during a mean follow-up period of 11 months (Fig. 2). Twelve (86%) out of 14 patients who underwent surgery alone showed complete occlusion. Seven (54%) of 13 patients who underwent GKS alone showed complete occlusion. Overall, angiographically confirmed complete occlusion was observed in 65 out of 95 DAVFs (68%). A summary of the treatment outcomes and modalities for DAVFs is shown in Table 5. The clinical outcome of DAVFs was excellent in 80 patients (84%). Fifteen patients had a GOS score of 4, and six of these patients received conservative treatment.
In the surgery, one patient with a DAVF located in the right parasagittal convexity developed hemiparesis just after a microsurgical interhemispheric approach. In the endovascular embolization, one patient with transverse-sigmoid sinus DAVF developed an intracranial hemorrhage during an interventional procedure. She gradually recovered and suffered no permanent neurological deficit due to subarachnoid hemorrhage and intraventricular hemorrhage. Two years after a combination of endovascular embolization and GKS, cerebellar hemorrhage occurred in one patient with a transverse-sigmoid sinus DAVF. Preoperative angiography showed a Borden type IIb DAVF draining into the transverse sinus and occlusion of the sigmoid sinus. Angiographic outcome at 12 months follow-up showed complete occlusion, however, cerebellar hemorrhage occurred during the follow-up period because embolization of the transverse sinus with coils eventually led to venous congestion of infratentorial veins, even though we thought the occluded sigmoid sinus useless. Another patient with a transverse-sigmoid sinus DAVF who received endovascular treatment and GKS developed facial palsy. Overall, complication rate of DAVF treatment was 4%.
Patients with DAVFs typically present with symptoms at a mean age of 50 to 60 years. The presentation is variable, depending on the characteristics of the venous outflow and the anatomic location.6)8)19) Many authors have reported that most DAVFs are located in the transverse, sigmoid, and cavernous sinuses and that these lesions have no gender preponderance; however, gender preponderance has been demonstrated in several studies.6)9)20) Similar to other reports, in this study, patients with DAVFs presented at a mean age of 59.2 years and the majority of lesions were located in the transverse-sigmoid and cavernous sinuses and had no gender preponderance.
Patients may be asymptomatic or may experience symptoms ranging from mild to aggressive, according to lesion location and pattern of venous drainage.17)21) Pulsatile tinnitus is a common symptom in patients with transverse-sigmoid sinus DAVFs, which results from increased blood flow through the dural venous sinuses close to the middle ear.8)36) Due to their proximity to the orbit, cavernous sinus DAVFs can present with ophthalmoplegia, proptosis, chemosis, retro-orbital pain, and decreased visual acuity.19)24)38) The ocular signs and symptoms could generally be reversible if not longstanding.1) DAVFs that drain into the SSS or deep venous system produce symptoms of global venous congestion and persistent intracranial hypertension, leading to intracranial hemorrhage and may manifest with aggressive symptoms, such as hydrocephalus, seizures, and dementia.7)8)15)16)24)26) Brainstem DAVFs can also present with aggressive symptoms, such as cranial neuropathies and/or quadriparesis.22)24)
In this study, the most common symptoms of cavernous sinus DAVF were ocular symptoms (40%) and aggressive neurologic symptoms were rare; there was no occurrence of intracranial hemorrhage. One patient with a cavernous sinus DAVF experienced sudden blindness associated with thrombosis of the central retinal artery. Borden types II and III were much more common in cavernous sinus DAVFs than in transverse-sigmoid sinus DAVFs (93% versus 61%, respectively), while transverse-sigmoid sinus DAVFs were more frequently associated with hemorrhage and NHND, compared with cavernous sinus DAVFs (Table 2). Suh et al. reported that, although a cavernous sinus DAVF has a high grade angiographic finding, it can have a benign nature because it has sufficient venous drainage routes.35) Therefore, we can consider conservative management in low grade and/or asymptomatic cavernous sinus DAVFs and the treatment purpose of cavernous sinus DAVF as a clinical improvement rather than an angiographic cure. In cases involving progressive neurological symptoms, particularly decreased visual acuity, and in the presence of CVR, aggressive treatment of cavernous sinus DAVFs must be considered.
On the other hand, aggressive neurological symptoms correlated well with the venous drainage pattern of transverse-sigmoid sinus DAVFs because these occurred only in Borden type II and III. In this study, besides the venous drainage pattern, the location of a transverse-sigmoid sinus was found to be a factor affecting intracranial hemorrhage. Therefore, due to the low rate of spontaneous regression and the relatively high rate of aggressive symptoms, all transverse-sigmoid sinus DAVFs are considered to require treatment.21)
Aggressive neurologic symptoms were also seen in the majority of SSS DAVFs (62%) and tentorial DAVFs (60%). Seizure and intracranial hemorrhage were observed in SSS DAVFs, while trigeminal neuralgia and seizure were observed in tentorial DAVFs. Because SSS DAVFs are frequently associated with restrictive changes of the SSS and tentorial DAVFs usually drain via the leptomeningeal vein, retrograde cortical venous drainage and aggressive symptoms are frequently observed.8)21) For achievement of a complete cure, due to the high risk of neurological symptoms, these DAVFs generally require aggressive treatment.
Results of this study have demonstrated that DAVFs without cortical venous drainage on digital subtraction angiography at the time of diagnosis have a low risk of intracranial hemorrhage or neurologic deficits (Table 3). However, in our study, in DAVFs with cortical venous drainage, a 23% hemorrhage rate and 41% aggressive symptom rate were suggested. Brown et al.4) pointed out that 2% of Borden type I, 40% of Borden type II, and 80% of Borden type III DAVFs presented with intracranial hemorrhage or neurologic deficit. In our study, 6% of Borden type I, 31% of Borden type II, and 77% of Borden type III DAVFs presented with aggressive symptoms including intracranial hemorrhage (Table 3). Therefore, aggressive neurologic symptoms showed strong correlation with CVR.
Spontaneous obliteration of DAVFs has been proposed in cases of low grade DAVF and/or cavernous sinus DAVF.2)34)30) In this study, three patients with drainage of DAVFs to the cavernous sinus showed spontaneous obliteration during mean follow-up periods of 17.8 months, even though one of these was Borden type II. Nevertheless, conservative treatment was not recommended if the patient had progressive symptoms or presence of CVR. Van Dijk et al.38) described patients with partially treated or conservatively followed DAVFs with CVR for a mean follow-up period of 4.3 years. At the last follow-up, 16 (80%) patients had developed intracerebral hemorrhage (25%) or ischemic deficits (66%) in DAVFs with persistent CVR. They advocated an annual risk of intracranial hemorrhage of 8.1%, and an annual risk of NHND of 6.9% in Borden type II and III.38) In our study, nine patients (9%) were conservatively followed and five of them were DAVFs with CVR. Although one patient with CVR had a GOS score of 5, four patients had recurrent or newly developed symptoms for a mean period of 16.6 months. However, the mean follow-up period in this study was too short to reflect the aggressive behavior of intracranial DAVFs with long-term persistence of the CVR. In addition to cases with increased rates of aggressive symptoms, appropriate treatment is required in cases showing the presence of CVR because persistent CVR can cause irreversible outcomes even after accomplishing complete occlusion (Fig. 2).
Fifty nine patients (62%) were treated with endovascular embolization and 20 of these were treated with embolization alone (Table 5). Due to its high cure rate and relatively low complication rate, transvenous embolization via the transfemoral approach is the first choice of treatment.14)33)49) In cases involving severe stenosis or occlusion of the sinus, transarterial embolization using liquid embolic materials is another option. In this study, 16 of 20 patients (80%) had complete occlusion in angiographic outcome and one patient had an intracranial hemorrhage related to the intervention. Consideration of the advantages and disadvantages of transarterial, transvenous, and combined approaches should be given in each case before proceeding with embolization.11)
When endovascular embolization is technically difficult or results in incomplete occlusion, surgical treatment or GKS is required. DAVFs in the anterior cranial fossa are more amenable to surgery because these DAVFs are supplied by the ophthalmic arteries, in which catheterization is difficult and dangerous.10)29) In this study, two patients with an anterior cranial fossa DAVF underwent successful surgery and achieved complete occlusion. Due to the difficult endovascular access route, combination therapy of embolization and surgery was performed in the SSS DAVF and the tentorial DAVF. In this study, complete occlusion using embolization and surgery was achieved in 80% of SSS DAVFs and 100% of tentorial DAVFs. Ushikoshi et al.37) reported that DAVFs with Borden type III can be treated by embolization and surgery with a high rate of cure. The efficacy of this combined approach for DAVF ablation has been contended at nearly 100%, however, the risk of morbidity and mortality remains considerable.10)12)18) We treated nine patients with Borden type II and III DAVFs using a combination of embolization and surgery, and achieved complete occlusion in 89% of them, without complications (Table 5).
In our study, 43 patients with DAVFs were treated by GKS or a combination of GKS and embolization. The early results from another report have been encouraging, with obliteration rates as high as 93% for combined endovascular embolization and GKS, but have also demonstrated obliteration rates as low as 50% when only GKS is used.32)39) The cumulative cure rate for cavernous sinus DAVFs approached 75% at 24 months when only GKS was used, which was much better than that of other intracranial DAVFs.39) In our study, the obliteration rate reached 54% when only GKS was used and 83% when GKS was combined with endovascular embolization (Table 5). Five patients with cavernous sinus DAVFs were treated with GKS alone and four (80%) had complete obliteration and showed improvement in clinical symptoms during the mean follow-up period of 32 months. In this study, although the number of patients was small, patients with cavernous sinus DAVF showed good outcomes with GKS alone. Lalwani et al.23), who recommended strategies for treatment of transversesigmoid sinus DAVFs according to a grading system based on the severity of venous restrictive disease, reported that 22 (88%) of 25 patients achieved both clinical and angiographic cure following an endovascular or/and surgical approach. However, recent studies on GKS for transverse-sigmoid sinus DAVFs showed a relatively high occlusion rate of the DAVF (approximately 60% of cases) several months after treatment, without significant complications.27)31) We treated a majority (70%) of transverse-sigmoid sinus DAVFs using GKS: Borden type I by GKS only or a combination of embolization and GKS and Borden type II and III by a combination of embolization and GKS. Complete angiographic occlusion was achieved in 82% of these patients and cerebellar hemorrhage occurred in only one patient during the follow-up period. In overall angiographic outcome, patients treated with endovascular embolization and GKS had complete occlusion of 83%. In our opinion, although we had little experience with GKS in other locations, except the transverse-sigmoid and cavernous sinus DAVF, the efficacy of GKS in treatment of cavernous sinus and transverse-sigmoid sinus DAVFs suggests that irradiation might be an effective treatment for DAVFs at other locations. However, because the risk of hemorrhage remains elevated until completion of vessel thrombosis, treatment with GKS alone currently remains limited, particularly in DAVFs with aggressive symptoms.13) Combination of GKS with other treatments can resolve this problem successfully, with few side effects. Therefore, GKS alone is inappropriate as the primary treatment in DAVFs with CVR, and should be considered as an option, especially in poor surgical or endovascular candidates.
The clinical characteristics of intracranial DAVF depend on the lesion location and venous drainage pattern. In our study, the hemorrhagic risk associated with intracranial DAVFs increased according to the severity of CVR, and the transverse-sigmoid sinus is the location that most affects intracranial hemorrhage.
For achievement of a successful treatment outcome of intracranial DAVFs, strategies for treatment of intracranial DAVFs should be decided according to the characteristics of the DAVF, based on the location and drainage pattern. Conservative treatment can be considered in low grade cavernous sinus DAVFs. Aggressive multidisciplinary treatment, using endovascular embolization, surgery, GKS, and combination therapy, is usually required in the presence of CVR and/or at locations other than the cavernous sinus. In this study, we had good treatment outcomes using GKS, compared with other treatment modalities. Therefore, we recommend GKS in addition to other treatment modalities as an alternative method for treatment of intracranial DAVFs in appropriately indicated cases.
Figures and Tables
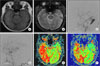
Fig. 1
42-year-old female patient with altered mentality and vomiting visited the emergency unit. Computed tomographic angiography (CTA) on initial assessment shows an acute intracerebral hemorrhage at the right temporo-parietal lobe and a mass effect. She underwent stereotactic hematoma evacuation and had no fixed neurological deficit (A). After six months, she revisited the emergency unit due to recurrent hemorrhage in the right temporo-parietal lobe. CT shows an intracerebral hematoma at the right temporo-parietal lobe and an intraventricular hemorrhage (B). Lateral view of an external carotid angiogram shows a transverse-sigmoid sinus dural arteriovenous fistula (DAVF) supplied by the middle meningeal artery and occipital artery (C). Lateral view of a common carotid angiogram obtained after glue embolization shows the remaining minimal DAVF. Glue embolization was followed by gamma knife stereotactic radiosurgery (GKS) because she had a DAVF with cortical venous reflux (CVR) only (D).

Fig. 2
A 64-year-old male patient presented with a chronic headache and pulsatile tinnitus. Magnetic resonance imaging (MRI) on initial assessment shows chronic cortical laminar necrosis and petechial hemorrhage, resulting from a previous venous infarction at the right temporo-occipital area (A, B). Lateral view of an external carotid angiogram shows a Borden type II, transverse-sigmoid sinus DAVF with occlusion of the sigmoid sinus and disturbed flow in the right transverse sinuses. Prominent cortical reflux is evident (C). Immediate postoperative assessment shows a complete fistula obliteration after transarterial embolization (D). The mean transit time (MTT) map on MRI shows a perfusion deficit in the right temporo-occipital lobe due to venous hypertension on preoperative assessment (E). At three months after embolization, the MTT map shows improved perfusion at the right temporo-occipital lobe. However, a regional prolonged MTT area remains at the right temporo-occipital lobe because of irreversible changes due to the venous infarction (F).
References
1. Sencer A, Kiris T. Intracranial dural arteriovenous fistulas: A brief review on classification and general features. Turk Neurosurg. 2006. 16(2):57–64.
2. Barrow DL, Spector RH, Braun IF, Landman JA, Tindall SC, Tindall GT. Classification and treatment of spontaneous carotid cavernous sinus fistulas. J Neurosurg. 1985. 02. 62(2):248–256.
3. Borden JA, Wu JK, Shucart WA. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg. 1995. 02. 82(2):166–179.

4. Brown RD Jr, Wiebers DO, Nichols DA. Intracranial dural arteriovenous fistulae: angiographic predictors of intracranial hemorrhage and clinical outcome in nonsurgical patients. J Neurosurg. 1994. 10. 81(4):531–538.

5. Castaigne P. [Rene Djindjian, 1918-1977]. Rev Neurol (Paris). 1977. 12. 133(12):736–738. French.
6. Chung SJ, Kim JS, Kim JC, Lee SK, Kwon SU, Lee MC, et al. Intracranial dural arteriovenous fistulas: analysis of 60 patients. Cerebrovasc Dis. 2002. 13(2):79–88.

7. Cognard C, Casasco A, Toevi M, Houdart E, Chiras J, Merland JJ. Dural arteriovenous fistulas as a cause of intracranial hypertension due to impairment of cranial venous outflow. J Neurol Neurosurg Psychiatry. 1998. 09. 65(3):308–316.

8. Cognard C, Gobin YP, Pierot L, Bailly AL, Houdart E, Casasco A, et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995. 03. 194(3):671–680.

9. Cognard C, Houdart E, Casasco AE, Jhaveri HS, Chapot R, Merland JJ. Connors JJ, Wojak JC, editors. Endovascular therapy and long term results for intracranial dural arteriovenous fistulae. Interventional Neuroradiology: Strategies and Practical Techniques. 1999. ed 1. Philadelphia: W.B. Saunders Co.;198–214.
10. Collice M, D'Aliberti G, Arena O, Solaini C, Fontana RA, Talamonti G, et al. Surgical treatment of intracranial dural arteriovenous fistulae: role of venous drainage. Neurosurgery. 2000. 07. 47(1):56–66. discussion 66-7.

11. Gandhi D, Chen J, Pearl M, Huang J, Gemmete JJ, Kathuria S. Intracranial dural arteriovenous fistulas: Classification, imaging findings, and treatment. AJNR Am J Neuroradiol. 2012. 06. 33(6):1007–1013.

12. Goto K, Sidipratomo P, Ogata N, Inoue T, Matsuno H. Combining endovascular and neurosurgical treatments of high-risk dural arteriovenous fistulas in the lateral sinus and the confluence of the sinuses. J Neurosurg. 1999. 02. 90(2):289–299.

13. Guo WY, Pan DH, Wu HM, Chung WY, Shiau CY, Wang LW, et al. Radiosurgery as a treatment alternative for dural arteriovenous fistulas of the cavernous sinus. AJNR Am J Neuroradiol. 1998. Jun-Jul. 19(6):1081–1087.
14. Halbach VV, Higashida RT, Hieshima GB, Mehringer CM, Hardin CW. Transvenous embolization of dural fistulas involving the transverse and sigmoid sinuses. AJNR Am J Neuroradiol. 1989. Mar-Apr. 10(2):385–392.
15. Hasumi T, Fukushima T, Haisa T, Yonemitsu T, Waragai M. Focal dural arteriovenous fistula (DAVF) presenting with progressive cognitive impairment including amnesia and alexia. Intern Med. 2007. 46(16):1317–1320.

16. Hirono N, Yamadori A, Komiyama M. Dural arteriovenous fistula: A cause of hypoperfusion-induced intellectual impairment. Eur Neurol. 1993. 33(1):5–8.
17. Hurst RW, Bagley LJ, Galetta S, Glosser G, Lieberman AP, Trojanowski J, et al. Dementia resulting from dural arteriovenous fistulas: the pathologic findings of venous hypertensive encephalopathy. AJNR Am J Neuroradiol. 1998. 08. 19(7):1267–1273.
18. Kakarla UK, Deshmukh VR, Zabramski JM, Albuquerque FC, McDougall CG, Spetzler RF. Surgical treatment of high-risk intracranial dural arteriovenous fistulae: clinical outcomes and avoidance of complications. Neurosurgery. 2007. 09. 61(3):447–457. discussion 457-9.
19. Kim MS, Han DH, Kwon OK, Oh CW, Han MH. Clinical characteristics of dural arteriovenous fistula. J Clin Neurosci. 2002. 03. 9(2):147–155.

20. Kirsch M, Liebig T, Kuhne D, Henkes H. Endovascular management of dural arteriovenous fistulas of the transverse and sigmoid sinus in 150 patients. Neuroradiology. 2009. 07. 51(7):477–483.

21. Kiyosue H, Hori Y, Okahara M, Tanoue S, Sagara Y, Matsumoto S, et al. Treatment of intracranial dural arteriovenous fistulas: current strategies based on location and hemodynamics, and alternative techniques of transcatheter embolization. Radiographics. 2004. Nov-Dec. 24(6):1637–1653.

22. Lagares A, Perez-Nunez A, Alday R, Ramos A, Campollo J, Lobato RD. Dural arteriovenous fistula presenting as brainstem ischaemia. Acta Neurochir (Wien). 2007. 149(9):965–967. discussion 967.

23. Lalwani AK, Dowd CF, Halbach VV. Grading venous restrictive disease in patients with dural arteriovenous fistulas of the transverse/sigmoid sinus. J Neurosurg. 1993. 07. 79(1):11–15.

24. Lasjaunias P, Chiu M, ter Brugge K, Tolia A, Hurth M, Bernstein M. Neurological manifestations of intracranial dural arteriovenous malformations. J Neurosurg. 1986. 05. 64(5):724–730.

25. Lawton MT, Chun J, Wilson CB, Halbach VV. Ethmoidal dural arteriovenous fistulae: an assessment of surgical and endovascular management. Neurosurgery. 1999. 10. 45(4):805–810. discussion 810-1.

26. Lee PH, Lee JS, Shin DH, Kim BM, Huh K. Parkinsonism as an initial manifestation of dural arteriovenous fistula. Eur J Neurol. 2005. 05. 12(5):403–406.

27. Lewis AI, Tomsick TA, Tew JM Jr. Management of tentorial dural arteriovenous malformations: transarterial embolization combined with stereotactic radiation or surgery. J Neurosurg. 1994. 12. 81(6):851–859.

28. Lucas Cde P, Zabramski JM. Dural arteriovenous fistula of the transverse-sigmoid sinus causing trigeminal neuralgia. Acta Neurochir (Wien). 2007. 12. 149(12):1249–1253. discussion 1253.

29. Lucas CP, Zabramski JM, Spetzler RF, Jacobowitz R. Treatment for intracranial dural arteriovenous malformations: a meta-analysis from the English language literature. Neurosurgery. 1997. 06. 40(6):1119–1130. discussion 1130-2.

30. Luciani A, Houdart E, Mounayer C, Saint Maurice JP, Merland JJ. Spontaneous closure of dural arteriovenous fistulas: report of three cases and review of the literature. AJNR Am J Neuroradiol. 2001. 05. 22(5):992–996.
31. Pan DH, Chung WY, Guo WY, Wu HM, Liu KD, Shiau CY, et al. Stereotactic radiosurgery for the treatment of dural arteriovenous fistulas involving the transverse-sigmoid sinus. J Neurosurg. 2002. 05. 96(5):823–829.

32. Pollock BE, Nichols DA, Garrity JA, Gorman DA, Stafford SL. Stereotactic radiosurgery and particulate embolization for cavernous sinus dural arteriovenous fistulae. Neurosurgery. 1999. 09. 45(3):459–466. discussion 466-7.

33. Roy D, Raymond J. The role of transvenous embolization in the treatment of intracranial dural arteriovenous fistulas. Neurosurgery. 1997. 06. 40(6):1133–1141. discussion 1141-4.

34. Sasaki H, Nukui H, Kaneko M, Mitsuka S, Hosaka T, Kakizawa T, et al. Long-term observations in cases with spontaneous carotid cavernous fistulas. Acta Neurochir (Wien). 1988. 90(3-4):117–120.
35. Suh DC, Lee JH, Kim SJ, Chung SJ, Choi CG, Kim HJ, et al. New concept in cavernous sinus dural arteriovenous fistula: correlation with presenting symptom and venous drainage patterns. Stroke. 2005. 06. 36(6):1134–1139.
36. Sung KH, Min KS, Lee MS, Kim YG, Kim DH. Treatment modalities for dural arteriovenous fistulas (DAVFs) according to venous drainage patterns. Korean J Cerebrovasc Surg. 2008. 06. 10(2):364–373.
37. Ushikoshi S, Houkin K, Kuroda S, Asano T, Iwasaki Y, Miyasaka K, et al. Surgical treatment of intracranial dural arteriovenous fistulas. Surg Neurol. 2002. 04. 57(4):253–261.





 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download