Abstract
Objective
Patients with severe spontaneous cerebellar hemorrhage typically undergo treatment with suboccipital craniectomy and hematoma evacuation. However, this is a stressful procedure for patients due to the long operating time and operation-induced tissue damage. In addition, the durotomy can result in pseudomeningocele. We investigated the efficacy of stereotactic or navigation-guided burr hole aspiration surgery as a treatment for spontaneous hypertensive cerebellar hemorrhage (SHCH).
Methods
Between January 2002 and December 2011, 26 patients with SHCH underwent surgery using the stereotactic or navigation-guided burr hole aspiration and catheter insertion technique in our institution.
Results
Mean hematoma volume was 21.8 ± 5.8 cc at admission and 13.1 ± 5.4 cc immediately following surgery. Preoperative Glasgow Coma Scale (GCS) score was 12.5 ± 1.3 and postoperative GCS score was 13.1 ± 1.2. Seven days after surgery, the mean hematoma volume was 4.3 ± 5.6 cc, and there was no occurrence of surgery-related complications during the six-month follow-up period. The mean operation time for catheter insertion was 43.1 ± 8.9 min, and a mean 31.3 ± 6.0 min was also added for extra-ventricular drainage. The mean Glasgow Outcome Scale (GOS) score after six months was 4.6 ± 1.0.
Conclusion
Stereotactic burr hole aspiration surgery for treatment of SHCH is less time-consuming and invasive than other interventions, and resulted in no surgery-related complications. Therefore, we suggest that this surgical method could be a safe and effective treatment option for selected patients with SHCH.
Spontaneous hypertensive cerebellar hemorrhage (SHCH) accounts for 5-10% of all cases of intracerebral hemorrhage (ICH).7) The mortality rate of SHCH is reportedly 20-75% higher than that of supratentorial ICH.4) This high mortality rate results from direct compression of the hematoma by the brain stem and acute hydrocephalus due to hematoma extension into the fourth ventricle or direct compression of the fourth ventricle.17)
In patients with a large volume SHCH, a large suboccipital craniectomy (SOC) is frequently used for hematoma evacuation.9)14) Due to the long operating time and operation-induced tissue damage, this procedure is particularly stressful for elderly patients. In addition, because of the large skin incision and durotomy required for the procedure, it can result in pseudomeningocele.
Because burr hole aspiration surgery is less invasive, we thought that it may result in improved clinical outcomes and lowered complication rates. The aim of this study is to investigate the clinical outcomes of patients treated with burr hole aspiration surgery using a stereotactic frame or navigation system.
We conducted a retrospective study of the medical records of 26 patients with SHCH who visited our institution between Jan 2002 and Dec 2011. All 26 patients who underwent stereotactic or navigation-guided burr hole aspiration surgery and catheter insertion were included in our study. On admission, all patients underwent standard neurological and clinical examinations, as well as routine laboratory tests. Diagnosis of SHCH was based on findings on computed tomography (CT). Some patients underwent CT angiography or magnetic resonance imaging in order to exclude aneurysm rupture, cavernous angioma, arteriovenous malformation, tumor, or hemorrhagic infarction.
In all patients, we evaluated variable factors, including the maximum diameter of the hematoma, the presence of hydrocephalus, brain stem compression, compression of the fourth ventricle, and the presence of intraventricular hemorrhage (IVH). The volume of the hematoma was measured by calculating the area occupied by the hyperdense hematoma on each slice of the brain CT images and multiplying the area by the thickness of each slice. We also evaluated patients using the Glasgow Coma Scale (GCS) on admission.
The indications for surgical treatment were provided by the American Heart Association/American Stroke Association (AHA/ASA). In short, surgery is recommended if the patient showed neurological deterioration and the maximum diameter of the hematoma was greater than 3 cm.2) (Fig. 1A) If the patient exhibited progressive mental deterioration associated with hydrocephalus caused by IVH or fourth ventricle obliteration, we performed external ventricular drainage (EVD). The Glasgow Outcome Scale (GOS) was used for assessment of patients' outcomes and complications were checked after six months.
Based on findings on the brain CT, we attempted to find the shortest trajectory targeting the hematoma center, and avoiding the venous sinus and midline. Under general anesthesia, the patients were placed in a military tuck position. Then, using the Leksell® Stereotactic System (Elekta Instrument AB, Stockholm, Sweden) or the Vectorvision® Navigation System (BrainLAB, Feldkirchen, Germany), we performed catheter insertion through the burr hole. An intradural catheter was placed in the center of the hematoma. Using a 10 cc syringe, careful manual aspiration of hematoma was performed with slightly negative pressure. The catheter was connected with a conventional cerebrospinal fluid (CSF) collection system. If the patient was satisfied with our EVD indication, we performed EVD through Kocher's point before aspiration surgery or through the posterior parietal point after aspiration surgery.
Each day following surgery, we examined the color and amount of the drained hematoma. If the volume of the hematoma was effectively drained every day, we checked brain CT at three days after surgery (Fig. 1B). If a substantial amount of hematoma still remained on the CT images, it was resolved with 5,000 IU and fibrinolyzed twice daily for two or three days, and the remaining hematoma was drained each day. In cases where the hematoma was especially thick and difficult to drain, we also used urokinase similarly for three days following the day after surgery. When the hematoma was not almost removed on follow-up brain CT, we drained the catheter for two additional days.
The study included seven males and 19 females. Age of patients ranged from 48 to 86 years. Burr hole catheter insertion using the stereotactic and navigation-guided systems was performed in 10 and 16 patients. The mean hematoma volume on brain CT at admission was 21.8 ± 5.8 cc, and the mean diameter of the hematomas on brain CT was 45.8 ± 8.7 mm. Seven of 26 patients had IVH and non-communicating hydrocephalus. The mean preoperative GCS score was 12.5 ± 1.3 and the mean postoperative GCS score was 13.1 ± 1.2.
In 19 cases, using only insertion of the catheter in the hematoma without EVD, the mean operation time was 43.1 ± 8.9 min. A mean 31.2 ± 6.0 min was added in the seven patients combined with EVD. The average volume of hematoma evacuated through the catheter during the operation was 9.3 ± 1.3 ml. Mean hematoma volume immediately following surgery was 13.1 ± 5.3 cc. On postoperative day three, residual mean hematoma volume was 8.7 ± 5.8 cc. However, in 14 cases, because the residual hematoma volume indicated by the brain CT exceeded 10 cc, we performed urokinase irrigation. We performed an EVD with placement of a catheter in seven cases and kept it in place for four days on average. On postoperative day seven, the mean residual hematoma volume was 4.3 ± 5.6 cc.
The mean follow-up duration was 23.7 ± 15.2 months and the GOS score was checked on all patients for an average of six months following surgery. Twenty three of the 26 patients showed good recovery (GR); one patient was mildly disabled (MD), one patient was severely disabled (SD), and one patient died (D). The overall mortality rate was 3.8% and the mean GOS score was 4.6 ± 1.0. The patient died of sudden myocardial infarction (MI); however, mental status had improved from deep drowsy to drowsy before occurrence of MI. Among 25 patients with a high GCS score over 10 points at admission, 23 patients showed the GR and displayed less or no severe brain stem compression. There was no occurrence of surgery-related complications, including re-bleeding, infection, and pseudomeningocele. A summary of patient characteristics and surgical results is shown in Table. 1.
Because it can compress the brainstem directly as well as generate acute hydrocephalus, SHCH is associated with high mortality rates. The indications for surgical intervention are generally clearer than in cases of supratentorial ICH. If certain diagnostic indications are satisfied, early operation for treatment of SHCH is strongly recommended.1)4)10) According to the 2007 AHA/ASA guidelines for management of ICH in adults,3) the surgical indication for SCH is patients with cerebellar hemorrhage > 3 cm who show neurological deterioration, or who have brain stem compression and/or hydrocephalus resulting from ventricular obstruction.
In cases involving acute massive bleeding, SOC is typically favorable for hematoma evacuation and brainstem decompression.3) SOC has the advantage of assuring effective decompression and direct hematoma evacuation, however, it requires long operation times and incurs additional risk from operation-induced tissue damage. Suboccipital craniectomy can also result in patient instability, additional brain injury, re-bleeding, pseudomeningocele due to durotomy, and wound infection in the postoperative period.15) Important factors affecting the prognosis of patients with cerebellar hemorrhage include the mental status of the patient at admission, a decrease in consciousness, the amount of hematoma with or without IVH, the presence of obstructive hydrocephalus and brain stem compression or obliteration of quadrigeminal cistern.5)6)8)11)15)16) Advantages of burr hole aspiration surgery include the short operation time, lowered risk of additional brain injury, small size of incision, lower complication rate, and the fact that it can be performed without durotomy. In our opinion, this operation is appropriate for patients who have mild brain stem compression, cannot tolerate long periods of operation, or have a poor medical condition or coagulopathy, as well as those at risk for fourth ventricle obstruction due to progression of brain swelling but still have patency of CSF flow.
In 1980, several institutions introduced stereotactic approaches for treatment of SHCH.12)13) According to Mohsen et al.12), who studied patients with good GCS scores (above 7), scores for all patients improved to above 11. In our study, among 25 patients with GCS scores of over 10, GCS score for all patients showed improvement to above 12. In addition, the overall mortality of patients was only 3.8%, and there was no occurrence of surgery-related complications. Even in the one case in which the patient passed away, the cause of death was a myocardial infarction rather than a complication of the SHCH or surgery.
However, this technique is restricted in the acute stage because direct hemostasis and confirmation of the source of the bleeding cannot be achieved.18) Re-bleeding may occur if stereotactic aspiration is performed too soon after the onset of hemorrhage.
The primary limitation of the current study is the lack of control groups and randomized multi-center case control series. Conduct of further research is required in order to validate our findings against these controls.
We suggest that, compared to conventional surgical approaches, aspiration surgery for treatment of SHCH can reduce the approaching route, operation time, and complication rates, such as injury of brain tissue and psudomeningocele. Therefore, it can be regarded as an alternative surgical option for treatment of SHCH in selected patients.
Figures and Tables
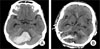 | Fig. 1
A 56-year-old male patient presented with a drowsy mental status. (A) Findings on brain Computed tomography (CT) at admission shows spontaneous cerebellar hemorrhage with mild brain stem compression and obstruction of the fourth ventricle. The hematoma volume was 26 cc and maximal hematoma diameter was 49 mm. (B) Brain CT at three days after surgery shows residual hematoma with catheter after stereotactic aspiration surgery. |
References
1. Brennan RW, Bergland RM. Acute cerebellar hemorrhage. Analysis of clinical findings and outcome in 12 cases. Neurology. 1977. 06. 27(6):527–532.

2. Broderick JP, Adams HP Jr, Barsan W, Feinberg W, Feldmann E, Grotta J, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 1999. 04. 30(4):905–915.
3. Broderick J, Connolly S, Feldmann E, Hanley D, Kase C, Krieger D, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Circulation. 2007. 10. 116(16):e391–e413.
4. Chin D, Carney P. Acute cerebellar hemorrhage with brainstem compression in contrast with benign cerebellar hemorrhage. Surg Neurol. 1983. 05. 19(5):406–409.

5. Donauer E, Loew F, Faubert C, Alesch F, Schaan M. Prognostic factors in the treatment of cerebellar hemorrhage. Acta Neurochir (Wien). 1994. 131(1-2):59–66.
6. Firsching R, Huber M, Frowein RA. Cerebellar hemorrhage: Management and prognosis. Neurosurg Rev. 1991. 14(3):191–194.
8. Kobayashi S, Sato A, Kageyama Y, Nakamura H, Watanabe Y, Yamaura A. Treatment of hypertensive cerebellar hemorrhage-Surgical or conservative management? Neurosurgery. 1994. 02. 34(2):246–250. discussion 250-1.

9. Koziarski A, Frankiewicz E. Medical and surgical treatment of intracerebellar hematomas. Acta Neurochir (Wien). 1991. 110(1-2):24–28.
10. Lui TN, Fairholm DJ, Shu TF, Chang CN, Lee ST, Chen HR. Surgical treatment of spontaneous cerebellar hemorrhage. Surg Neurol. 1985. 06. 23(6):555–558.

11. Mezzadri JJ, Otero JM, Ottino CA. Management of 50 spontaneous cerebellar hemorrhages. Importance of obstructive hydrocephalus. Acta Neurochir (Wien). 1993. 122(1-2):39–44.
12. Mohadjer M, Eggert R, May J, Mayfrank L. CT-guided stereotactic fibrinolysis of spontaneous and hypertensive cerebellar hemorrhage: long term results. J Neurosurg. 1990. 08. 73(2):217–222.
13. Niizuma H, Suzuki J. Computed tomography-guided stereotactic aspiration of posterior fossa hematomas: a supine lateral retromastoid approach. Neurosurgery. 1987. 09. 21(3):422–427.

14. Norris JW, Eisen AA, Branch CL. Problems in cerebellar hemorrhage and infarction. Neurology. 1969. 11. 19(11):1043–1050.

15. Salazar J, Vaquero J, Martinez P, Santos H, Martinez R, Bravo G. Clinical and CT scan assessment of benign versus fatal spontaneous cerebellar hematomas. Acta Neurochir (Wien). 1986. 79(2-4):80–86.
16. Taneda M, Hayakawa T, Mogami H. Primary cerebellar hemorrhage. Quadrigeminal cistern obliteration on CT scans as a predictor of outcome. J Neurosurg. 1987. 10. 67(4):545–552.
17. Winn HR. Youmans Neurological Surgery. 2011. ed 6. Philadelphia: W.B. Saunders Co;3712.
18. Yanaka K, Meguro K, Fujita K, Narushima K, Nose T. Immediate surgery reduces mortality in deeply comatose patients with spontaneous cerebellar hemorrhage. Neurol Med Chir (Tokyo). 2000. 06. 40(6):295–299. discussion 299-300.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download