Abstract
Objective
The limitations of medical management of symptomatic intracranial arterial stenosis (ICS) have prompted development of new strategies, including endovascular treatment. However, stenting of symptomatic ICS remains investigational. Here, we have reported and analyzed a series of 19 endovascular procedures involving placement of a Wingspan stent.
Methods
We conducted a retrospective review of a series of ICS in which patients were treated with percutaneous transarterial balloon angioplasty and stent placement (PTAS). Patients included in the study were diagnosed as symptomatic ICS between May 2010 and September 2011.
Results
Nineteen patients (median age, 65 years; 12 males, seven women) were treated with the Wingspan stent system for symptomatic ICS ranging from 50% to 99%. The technical success rate was 100%. The location of ICS included the internal carotid (n = 5; 1 petrous, 3 cavernous, and 1 clinoid segments), vertebral (n = 1; V4 segment), basilar (n = 1), and middle cerebral (n = 12; 9 M1, 3 M2) arteries. There was no occurrence of procedure-related mortality. Periprocedural morbidity occurred in two cases (10.5%), including carotid-cavernous fistula (n = 1) and subarachnoid hemorrhage (n = 1). No ipsilateral stroke was recorded beyond 30 days during a mean follow-up period of 13.2 months (range 9-19 months). Restenosis (> 50%) was observed in one patient (6.3%), who was asymptomatic, on follow-up imaging.
With widespread access to magnetic resonance imaging (MRI), computed tomographic angiography (CTA), and cerebral angiography, intracranial arterial stenosis (ICS) is increasingly diagnosed as a cause of cerebral ischemia. In the United States, 8 to 10% of ischemic strokes are attributed to ICS.4)17)23) ICS accounts for an even larger proportion of the disease in Asian countries, and is found in 22 to 26% of patients who suffer ischemic stroke.4)17)23) The risk of recurrent ischemic stroke in patients with ICS is estimated to be approximately 15% per year.3)21) According to findings of the recent Warfarin Aspirin Symptomatic Intracranial Disease (WASID) trial,8) despite medical treatment with warfarin or aspirin, ipsilateral vascular territory stroke occurred in 25% of patients with 70-99% stenosis and 11% of patients with 50-69% stenosis within two years.
These limitations to medical management of symptomatic ICS have prompted development of new strategies, including endovascular treatment. Percutaneous transarterial balloon angioplasty (PTA) without stent implantation was initially attempted, however, there was a risk of peri- and post-procedural vascular occlusion caused by elastic rebound or recoil or arterial dissection.6)13)14) Therefore, PTA with stenting for symptomatic ICS is now under investigation. According to findings of the Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial,16) there was a high risk of stroke and death within 30 days (14.7% vs 5.8%) after percutaneous transarterial balloon angioplasty and stent placement (PTAS) using the Wingspan stent. However, other studies have reported good results, indicating that PTAS were performed using the Wingspan stent.5)7) We also focused on the technical success and periprocedural complication rates of PTAS using this device in a series of 19 endovascular procedures using the Wingspan stent system.
Between May 2010 and September 2011, 19 patients in our institution with 50-99% ICS in 19 vessels were enrolled in this study. All patients underwent PTAS using the Wingspan stent system (Striker/Boston Scientific, Fremont, USA). Each patient had experienced ipsilateral transient ischemic attack (TIA) and/or stroke believed to be secondary to embolus or hemodynamic compromise related to the ICS lesion. Patient characteristics, lesions, and periprocedural complications were recorded. Success was defined upon completion of Gateway balloon angioplasty with placement of a Wingspan stent in the target vessel, and cases with some degree of residual stenosis (< 50%) or procedure-related complications were still deemed successful.
To reduce the risk of periprocedural thrombosis and restenosis, aspirin (100 mg) or clopidogrel (75 mg) therapy was initiated in all patients at least five days before the procedure or patients were given 400 mg of aspirin and 300 mg of clopidogrel one day before stent placement. Patients also received 80 mg of atorvastatin at least one day before the procedure. During the procedure, all patients received bolus 5,000 IU intravenous (IV) heparin. Protamine sulfate was readily available for treatment of hemorrhagic complications.
After administration of local anesthesia, we injected IV 2 mg/hour of nimodipine to prevent vasospasm. The right common femoral artery was cannulated and the 6F catheter access system was introduced and advanced into the targeted supra-aortic vessel. Three-dimensional (3D) angiographic images were obtained in order to evaluate the characteristics of the target vessel, including the proximal and distal diameters of the parent vessel and the diameter and length of the stenotic lesion. A guidewire was passed through the stenotic lesion and the microcatheter was introduced over the guidewire; the guidewire was then exchanged for a 0.014-inch microwire. The Gateway angioplasty balloon and Wingspan stent were advanced and the microcatheter was removed. In each case, the balloon length was chosen to match the length of the stenotic lesion, and the balloon diameter was approximately 80% of that of the parent artery. The balloon was advanced over the microwire until the stenotic lesion was completely covered. Angioplasty was performed by gradual inflation of the balloon at a pressure not greater than the burst pressure indicated on the product label. After deflation and withdrawal of the balloon, conventional angiography was performed to confirm dilatation of the stenotic lesion. Then, the self-expanding Wingspan stent was advanced into the area of the lesion and deployed. A stent with a diameter slightly larger than that of the parent artery and a length sufficient to cover the stenotic lesion and including an excess of a few millimeters on either side of the stenotic lesion was chosen. After deployment of the stent, a final angiogram was obtained; if residual stenosis was observed, additional balloon angioplasty was performed in 13 cases.
All patients were admitted to an intensive care unit for approximately one day, and then transferred to the general ward until it was assured that there were no complications. After discharge, all patients were instructed to take 100 mg aspirin, 75 mg clopidogrel, and 10 mg atorvastatin daily for six months.
Follow-up CTA studies were available for 15 patients with 16 stenotic lesions at approximately six months or later and cerebral angiography studies were available for 10 patients with 11 stenotic lesions at approximately one year or later. The aim of these follow-up studies was to check for restenosis exceeding 50% luminal narrowing of the treated vessels.
A total of 19 patients underwent PTAS for 19 stenotic arteries. A summary of patient data is shown in Table 1. The median age of patients was 65 years (range 53-74 years), and there were 12 males and seven females. Eleven of 19 patients (57.9%) were found to have an ipsilateral infarction related to the stenotic lesion confirmed by MRI, and eight patients (42.1%) had experienced TIA referable to the stenotic lesion. The location of ICS included the internal carotid (n = 5; 1 petrous, 3 cavernous, and 1 clinoid segments), vertebral (n = 1; V4 segment), basilar (n = 1), and middle cerebral (n = 12; 9M1, 3M2) arteries. We enrolled patients with stenosis exceeding 50%; the initial median stenosis was 63% (range, 52-99%). Thirteen of 19 patients were mild stenosis (range 50-69%), and six of 19 patients were severe stenosis (70-99%). After the procedure, successful reduction (< 50%) was achieved in all patients, and final post procedural angiography indicated increasing blood flow to the arteries distal to the stent implantation site. The periprocedural complications reported in two cases included occlusion due to thrombosis followed by carotid-cavernous fistula (CCF) and subarachnoid hemorrhage (SAH) presumably due to vessel injury. There was no occurrence of procedure-related mortality.
All patients were available for initial follow-up with CTA at a median time of seven months (range 3-11 months). One of these patients had an asymptomatic restenosis of the right MCA of approximately 45%. At a median time of 14 months (range, 9-19 months) post-procedure, 16 of 19 patients were available to undergo cerebral angiography, while three were lost to follow up. One patient (6.3%) had developed restenosis of approximately 60% by 14 months and underwent repeat balloon angioplasty. After the procedure, the patient remained asymptomatic, without TIA or stroke at regular follow-up.
A 61-year old male patient presented with memory impairment and transient left hemiparesis. He had a history of treatment for hypertension for approximately one year. MRI findings were consistent with an old infarct of the right periventricular white matter, and magnetic resonance angiography indicated severe stenosis at M1 and M2. Cerebral angiography confirmed right M1 stenosis (60%) with ulceration and 90% of M2 stenosis. We planned PTAS for the M1 lesion, and, due to the small M2 diameter, PTA for the M2 lesion. Balloon angioplasty was performed using a gateway balloon (2.25 × 15 mm) for the M1 stenosis, followed by placement of a 3 × 22 mm Wingspan stent. Balloon angioplasty (1.5 × 15 mm) was then performed for the M2 stenosis. Initial imaging suggested successful reduction of both lesions. The next day, the patient showed deterioration and developed a gradually worsening headache. Brain CT showed SAH in the right frontal and temporal area, however, clinically there was no focal neurological deficit. The patient received conservative management, therefore, the hemorrhage showed complete resolution, and the headache showed improvement. On six-month follow up CTA, there was no evidence of restenosis in both the M1 and M2 lesions.
A 64-year old female patient presented with transient dizziness and memory impairment. She had a history of previous ischemic stroke with a 10-year medical history of diabetes mellitus and hypertension. Findings on MRI revealed an old border zone infarct at the right fronto-parietal lobe, and old small infarctions at the basal ganglia and the right paramedian pons. Cerebral angiography indicated right cavernous ICA stenosis of approximately 75%. During insertion of guiding catheter, acute thrombosis occurred and the patient developed left hemiparesis (motor grade 2). After thrombolysis with 300,000 IU urokinase, the occluded artery was recanalized and the left hemiparesis showed improvement, allowing us to perform balloon angioplasty using a Gateway balloon (2.5 × 15 mm) with successful placement of a 4 × 20 mm Wingspan stent. We obtained post-procedure images indicating reduction of stenosis (stenosis 25%). However, cerebral angiography revealed CCF at the stent implantation site. Transvenous coil embolization was performed successfully through the right inferior petrosal sinus. After three days, the patient developed third nerve palsy, including ptosis and paralysis of extraocular movement, however, diffusion MRI did not reveal any abnormalities. The third nerve palsy showed gradual improvement, and showed complete resolution within three months.
Medical therapy with warfarin or aspirin was unsatisfactory for prevention of recurrent stroke, brain hemorrhage, or death in ICS patients.8)21) Several alternative strategies for revascularization, including surgical bypass, PTA alone, and PTAS, have been advanced. Despite good patency rates after surgical bypass, approximately 96% of bypass surgeries are still found to yield no benefit at long term follow up, when compared with medical treatment.15)19)
Since the introduction of single lumen balloon microcatheters in 1992, PTA alone for intracranial stenosis has been used in some centers, and outcomes have compared favorably to those of medical treatment.1)11)12) In a study reported by Mark et al.,13) the 30-day rate for stroke and death was 5.8%, and the annual rate of stroke in the ipsilateral territory of the stenotic lesion was 3.2%. However, residual stenosis, periprocedural morbidity, and restenosis should also be considered; in the same study, 40.7% of patients who were treated with PTA alone had residual stenosis of > 50%. To reduce the rate of residual stenosis, sufficient balloon inflation is needed, which can increase the risk of periprocedural morbidity, including dissection, arterial rupture, and distal embolization. Major periprocedural morbidity was reported in up to 50% of cases, with a fatality rate of 17%.6) Among the morbidities, the incidence of vessel dissection was 20.2%, although, in some cases, this has been repaired with immediate stent implantation.13) In addition, because PTA alone may have poor durability, the rate of restenosis is relatively high. In a nonrandomized single center database review, the rate of restenosis after PTA alone was 50%.14)
In treatment of coronary artery stenosis, PTAS showed better results than PTA alone.2)18) Therefore, PTAS became a standard treatment option in treatment of coronary artery stenosis. Thereafter, to avoid variable outcomes of PTA alone for treatment of cerebral lesions, primary PTAS was introduced. In the SSYLVIA (Stenting of Symptomatic Atherosclerotic Lesions in the Vertebral or Intracranial Arteries) study,20) use of balloon expandable stents resulted in delivery of more ideal results than PTA alone. At six-month follow up evaluations, only 32% of patients with intracranial arterial stenosis had a > 50% restenosis, and the stroke rate during the first 30 post-procedural days was 6.6%, with an additional stroke rate of 7.3% thereafter. However, several problems were identified, including vessel dissection, perforation, mainly due to over-sizing of the stent, and the "dog-bone" effect of the balloon. To reduce these complications, intentional under-dilatation has become a widely accepted strategy; however, under-dilatation has resulted in stents that are not fixed to the vessel wall and can therefore be movable within the dilated vessel.7)
Into this scenario came the Wingspan stent delivery system which claimed several advantages. Because these stents are self-expanding, over-dilatation of the vessel during balloon inflation is not necessary. For this reason, a reduction in the number of balloon dilatation-induced vessel injuries, such as perforation, dissection, and distal embolization was anticipated. In the present study, two of 19 patients (10.5%) had a vessel injury (case 1, 2). However, one (case 2) vessel injury was not actually associated with balloon inflation, but was due to a guidewire-induced injury. Because we did not perform follow-up MRIs, we reviewed other studies reporting on distal embolization seen on MRI. In a study of PTAS using the Wingspan stent, the incidence of distal embolization on diffusion MRI was 34.2%, and 70% in a study of PTAS using the balloon expandable stent.5)22) Advantages of the Wingspan system include a highly flexible microcatheter delivery system that is trackable to facilitate access through relatively tortuous cerebrovascular vessels and a round-tip microcatheter to facilitate atraumatic catheter placement. These features help to avoid unnecessary vessel distortion and reduce risk of spasm and dissection of parent vessels. The highly flexible nature also prevents accidental guidewire withdrawal during removal of the balloon catheter and stent delivery, as well as unnecessarily aggressive distal microwire delivery. Finally, because the Wingspan stent is designed to be self-expandable and highly flexible, it promotes vessel wall apposition in curved and tapered vessels and contributes to a potentially high rate of success. The stent features a flexible open cell design for enhanced conformability and access, particularly with longer stent lengths. In addition, in the long term, the self-expandable feature interrupts periprocedural elastic recoil as well as restenosis. In our study, the rate of successful stent implantation was 100%. Restenosis occurred in one of 16 patients (6.5%) during a one-year follow up period, and there were two periprocedural complications. Further data collection and continued long-term follow up will be necessary in order to evaluate exact restenosis and long-term patency rates.
To reduce the risk of periprocedural complications, we addressed several considerations in our PTAS procedures. In selection of the target vessel, there must be careful tension while advancing the guiding catheter and inflating the balloon. In addition, we recommend use of high dose statins in patients undergoing PTAS for ICS. In stenting for myocardial infarction (MI), a lower rate of periprocedural complications and 30-days adverse events was observed in patients who received high dose statins (80 mg atorvastatin) than in those who received conventional dose statins (10 mg atorvastatin) (5.8 vs 10.6%).9) As in stenting for MI, we believe that use of high dose statins in stenting for cerebral stenosis might result in a reduction of periprocedural complications.
The present study is limited by the relatively small number of patients, and more data with longer follow-up are needed in order to more thoroughly evaluate the efficacy of PTAS.
PTAS for symptomatic ICS can be safely and successfully performed using the Gateway balloon Wingspan stent system. Our initial experience suggests the potential for this procedure as a viable treatment option with a high rate of technical success and acceptable periprocedural morbidity for this patient population.
Figures and Tables
Fig. 1
(A) Cerebral angiography shows M1 (black arrow) and M2 (open arrow) stenosis (60% and 90%). (B) Cerebral angiography after percutaneous transarterial balloon angioplasty and stent placement (PTAS) with a Wingspan stent for M1 stenosis and percutaneous transarterial balloon angioplasty (PTA) for M2 stenosis shows successful reduction of the stenosis. (C) Follow up computed tomography (CT) image shows subarachnoid hemorrhage (SAH) in the right fontal and temporal area.
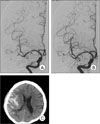
Fig. 2
Antero-posterior (A) and lateral (B) cerebral angiography shows 75% stenosis of the right cavernous internal carotid artery (ICA). (C) PTAS was performed successfully. Some degree of residual stenosis is shown (~25%). (D) Cerebral angiography shows a carotid-cavernous fistula (CCF) after PTAS. (E) After coil embolization, cerebral angiography shows near total occlusion of the CCF.

References
1. Berg-Dammer E, Henkes H, Weber W, Berlit P, Kuhne D. Percutaneous transluminal angioplasty of intracranial artery stenosis: clinical results in 24 patients. Neurosurg Focus. 1998. 10. 5(4):e13.

2. Betriu A, Masotti M, Serra A, Alonso J, Fernández-Avilés F, Gimeno F, et al. Randomized comparison of coronary stent implantation and balloon angioplasty in the treatment of de novo coronary artery lesions (START): a four-year follow-up. J Am Coll Cardiol. 1999. 11. 34(5):1498–1506.
3. Chimowitz MI, Kokkinos J, Strong J, Brown MB, Levine SR, Silliman S, et al. The warfarin-aspirin symptomatic intracranial disease study. Neurology. 1995. 08. 45(8):1488–1493.

4. Feldmann E, Daneault N, Kwan E, Ho KJ, Pessin MS, Langenberg P, et al. Chinese-white differences in the distribution of occlusive cerebrovascular disease. Neurology. 1990. 10. 40(10):1541–1545.

5. Fiorella D, Levy EI, Turk AS, Albuquerque FC, Niemann DB, Aagaard-Kienitz B, et al. US multicenter experience with the wingspan stent system for the treatment of intracranial atheromatous disease: periprocedural results. Stroke. 2007. 03. 38(3):881–887.

6. Gupta R, Schumacher HC, Mangla S, Meyers PM, Duong H, Khandji AG, et al. Urgent endovascular revascularization for symptomatic intracranial atherosclerotic stenosis. Neurology. 2003. 12. 61(12):1729–1735.

7. Henkes H, Miloslavski E, Lowens S, Reinartz J, Liebig T, Kühne D. Treatment of intracranial atherosclerotic stenoses with balloon dilatation and self-expanding stent deployment (WingSpan). Neuroradiology. 2005. 03. 47(3):222–228.

8. Kasner SE, Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation. 2006. 01. 113(4):555–563.

9. Kim JS, Kim J, Choi D, Lee CJ, Lee SH, Ko YG, et al. Efficacy of high-dose atorvastatin loading before primary percutaneous coronary intervention in ST-segment elevation myocardial infarction: the STATIN STEMI trial. JACC Cardiovasc Interv. 2010. 03. 3(3):332–339.
10. Lylyk P, Cohen JE, Ceratto R, Ferrario A, Miranda C. Angioplasty and stent placement in intracranial atherosclerotic stenoses and dissections. AJNR Am J Neuroradiol. 2002. 03. 23(3):430–436.
11. Marks MP, Marcellus ML, Do HM, Schraedley-Desmond PK, Steinberg GK, Tong DC, et al. Intracranial angioplasty without stenting for symptomatic atherosclerotic stenosis: long-term follow-up. AJNR Am J Neuroradiol. 2005. 03. 26(3):525–530.
12. Marks MP, Marcellus M, Norbash AM, Steinberg GK, Tong D, Albers GW. Outcome of angioplasty for atherosclerotic intracranial stenosis. Stroke. 1999. 05. 30(5):1065–1069.

13. Marks MP, Wojak JC, Al-Ali F, Jayaraman M, Marcellus ML, Connors JJ, et al. Angioplasty for symptomatic intracranial stenosis: clinical outcome. Stroke. 2006. 04. 37(4):1016–1020.
14. Mazighi M, Yadav JS, Abou-Chebl A. Durability of endovascular therapy for symptomatic intracranial atherosclerosis. Stroke. 2008. 06. 39(6):1766–1769.

15. Powers WJ, Clarke WR, Grubb RL Jr, Videen TO, Adams HP Jr, Derdeyn CP, et al. Extracranial-intracranial bypass surgery for stroke prevention in hemodynamic cerebral ischemia: the Carotid Occlusion Surgery Study randomized trial. JAMA. 2011. 11. 306(18):1983–1992.
16. Rahme RJ, Aoun SG, Batjer HH, Bendok BR. SAMMPRIS; end of intracranial stenting for atherosclerosis or back to the drawing board? Neurosurgery. 2011. 12. 69(6):N16–N18.
17. Sacco RL, Kargman DE, Gu Q, Zamanillo MC. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study. Stroke. 1995. 01. 26(1):14–20.
18. Suryapranata H, van't Hof AW, Hoorntje JC, de Boer MJ, Zijlstra F. Randomized comparison of coronary stenting with balloon angioplasty in selected patients with acute myocardial infarction. Circulation. 1998. 06. 97(25):2502–2505.

19. The EC/IC Bypass Study Group. Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. N Engl J Med. 1985. 11. 313(19):1191–1200.
20. The SSYLVIA study investigators. Stenting of symptomatic atherosclerotic lesions in the vertebral or intracranial arteries (SSYLVIA): Study results. Stroke. 2004. 06. 35(6):1388–1392.
21. The Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) Study Group. Prognosis of patients with symptomatic vertebral or basilar artery stenosis. Stroke. 1998. 07. 29(7):1389–1392.
22. Tsumoto T, Terada T, Tsuura M, Matsumoto H, Masuo O, Yamaga H, et al. Diffusion-weighted imaging abnormalities after percutaneous transluminal angioplasty and stenting for intracranial atherosclerotic disease. AJNR Am J Neuroradiol. 2005. 02. 26(2):385–389.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download