Abstract
Objective
A retrospective review of premedication method and drug resistance of aspirin and clopidogrel in association with thromboembolic events during and after coil embolization of an unruptured intracranial aneurysm was conducted.
Methods
Our premedication policy for coil embolization of an unruptured intracranial aneurysm has changed from administration of the loading dose before the procedure (i.e. loading group) to repeated administration of the maintenance dose for several days (i.e. preparation group). The loading group (27 patients with 29 aneurysms) and the preparation group (30 patients with 35 aneurysms) were compared for identification of the effect of premedication method on periprocedural thromboembolic events. The results of drug response assays of the preparation group were analyzed with respect to periprocedural thromboembolic events.
Results
No statistically significant difference in incidence of thromboembolic events was observed between the loading group and the preparation group. Analysis of the results of the drug response assay showed high prevalence (56.7%, 73.3%) of clopidogrel resistance and relatively low prevalence (6.7%) of aspirin resistance. Patients who had thromboembolic events tended to have lower responsiveness to both aspirin and clopidogrel than patients without it.
Conclusion
The method of antiplatelet premedication does not affect the rate of periprocedural thromboembolic events in coil embolization for treatment of an unruptured intracranial aneurysm. Nevertheless, considering the high prevalence of drug resistance, it is reasonable to premedicate antiplatelet agents in the preparation method for the drug response assay. Use of a higher dose of aspirin and clopidogrel or addition of an alternative drug (cilostazol or triflusal) can be applied against antiplatelet agent resistance. However, because the hemorrhagic risk associated with this supplementary use of antiplatelet agent has not been well-documented, the hemorrhagic risk and the preventive benefit must be weighed.
The incidence of overall complication during elective coil embolization for treatment of unruptured aneurysms is low.26) Thromboembolic events account for the majority of complications, with a reported incidence of 3.4-6.2%.7)11)27) Antiplatelet agent premedication has been proposed for reducing thromboembolic risk, and the current evidence supports this preventive usage.3)7)11) Aspirin and clopidogrel have been widely used for antiplatelet premedication for endovascular procedures.4) Responsiveness to both drugs is quite variable, and there is a group of patients who exhibit low responsiveness to these two drugs.11)21) Aspirin and clopidogrel resistance have been known to show an association with thromboembolic events during the endovascular procedure.4)24) However, there is no general consensus on defining aspirin and clopidogrel resistance, which hinders investigation of the prevalence. Several studies have reported a prevalence of drug resistance of 5.5-60% for aspirin and 5-44% for clopidogrel.4)5)25)
Two methods for premedicating antiplatelet agents have been used; one is dosing the drugs for several days until the steady drug level is achieved and the other is to load a high-dose of the drugs before the procedure. The former can be described as a 'preparation method', while the latter can be called a 'loading method'. Little has been reported regarding the effects of these two premedication methods in the context of coil embolization for treatment of an unruptured aneurysm. In this study, we attempted to compare the difference in periprocedural thromboembolic events between the preparation method, in which patients take aspirin and clopidogrel for several days, and the loading method, in which patients take a high dose of aspirin and clopidogrel before the procedure.
Patients in the preparation group underwent drug response assays of clopidogrel and aspirin before the procedure. We analyzed the results of these drug response assays in relation to the periprocedural thromboembolic events. Literature on antiplatelet agent resistance was reviewed in the search for proper treatment for aspirin and clopidogrel resistance.
Subjects of this study underwent elective coil embolization for treatment of an unruptured intracranial aneurysm from January 2011 to June 2012. Our policy on antiplatelet agent premedication had changed from the loading method to the preparation method by the end of December 2011. In the loading method, 162 mg of aspirin and 300 mg of clopidogrel were administered orally on the day before the coil embolization procedure. In the preparation method, 81 mg of aspirin and 75 mg of clopidogrel were given per os in the morning for five days before the coil embolization procedure. On the day of coil embolization, 81 mg of aspirin and 75 mg of clopidogrel were given to all patients regardless of the premedication method. Patients were designated to either the 'Loading group' or the 'Preparation group' according to their method of taking antiplatelet agent before coil embolization. This designation was done in a consecutive manner.
In order to rule out the risk of stent insertion per se, patients who underwent stent-assisted coil embolization were excluded. Because it could be a significant independent risk factor, patients with a history of ischemic stroke and/or a higher than moderate degree of stenosis in any of the intracranial arteries and carotid arteries were also excluded. There were two intra-procedural ruptures of aneurysm, which were excluded, considering that vasospasm could be an interfering factor. Patients in both groups who were on antiplatelet therapy other than aspirin and clopidogrel were excluded, and, in order to avoid compounding by existing antiplatelet effects, patients who were already on aspirin, clopidogrel, or both were excluded from the loading group. Ultimately, 30 patients with 35 intracranial aneurysms were included in the preparation group, and 27 patients with 29 aneurysms were included in the loading group.
Thromboembolic events were categorized according to either radiological events or symptomatic events. Thrombus formation during the procedure, or any acute infarct on postoperative magnetic resonance images (MRI) were defined as radiological events. In the case of intraprocedural thrombus formation, glycoprotein IIb/IIIa inhibitor was administered through an intra-arterial route in all cases. Postoperative MRI was taken when there were any intraprocedural events, such as coil loop protrusion or any other suspicion of thromboembolism on the angiography. Symptomatic events were defined as any occurrence of newly-developed neurologic deficit or cerebral infarct-related symptoms, including lethargy, headache, dizziness and/or nausea after the coil embolization procedure. Visual impairment due to retina ischemia was also regarded as neurologic deficit. Patients who exhibited both symptoms and radiological finding of a thromboembolic event were regarded as having a symptomatic event.
In the preparation group, drug response assays for aspirin and clopidogrel were performed using the VerifyNow® system (Accumetrics, San Diego, CA). Aspirin reaction unit (ARU) was measured by VerifyNow® Rapid platelet function assay-aspirin (RPFA-ASA). P2Y12 reaction unit (PRU) and platelet inhibition rate were obtained from the VerifyNow® P2Y12 assay. Aspirin resistance was defined as ARU > 550 and clopidogrel resistance was defined as PRU > 230 or platelet inhibition rate < 20% referring to the manufacturer. When antiplatelet resistance was confirmed, either 600 mg of triflusal or 200 mg of cilostazol was administered before the coil embolization procedure. For administration of an alternative drug, platelet inhibition rate < 20% was used as the criteria for clopidogrel resistance.
SPSS version 16 for Windows (SPSS Inc, Chicago, IL) was used for statistical analysis. Continuous data were presented as mean ± standard deviation (SD) and were compared using the Mann-Whitney test. Fisher's exact test or chi-square test was used for processing of categorical data. Statistical significance was defined as 'p < 0.05'.
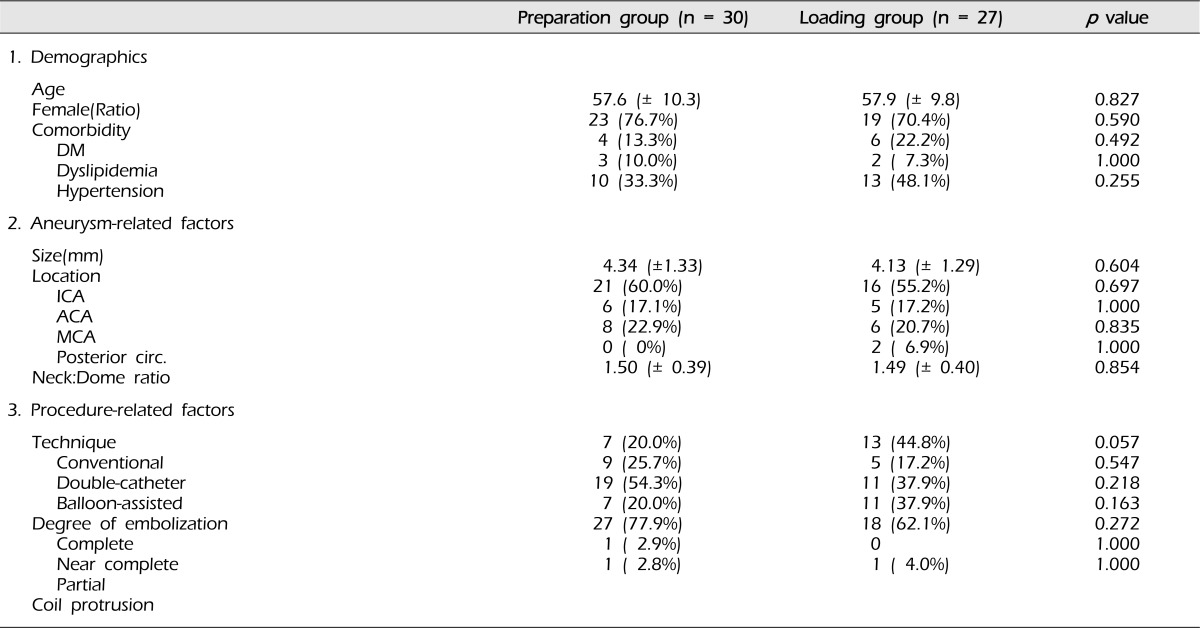
A summary of the basic characteristic of patients and aneurysms, and the results of coil embolization is shown in Table 1. No significant differences were noted in demographics, aneurysmal factors, and procedure-related factors. Mean age was 57.6 (± 10.3) years old in the preparation group and 57.9 (± 9.8) years old in the loading group. Female predominance was observed in both groups. Aneurysm size was 4.34 (± 1.33) mm in the preparation group and 4.13 (± 1.29) mm in the loading group. Neck: Dome ratios were similar in both groups. The most common location of the aneurysm was the ICA, followed by the MCA, in both groups, and ICA aneurysms consisted of more than half of all aneurysms. Although the difference was not statistically significant, more aneurysms were treated by a balloon-assisted technique in the preparation group, while more aneurysms were embolized by a conventional technique in the loading group. Most coil embolizations were either complete or near complete, with minimal neck remnant; 97.1% and 100% in the preparation group and the loading group, respectively.
The incidences of both radiological thromboembolic events and symptomatic thromboembolic events did not differ significantly between the preparation group and the loading group (Table 2). The overall incidence of thromboembolic events did not differ significantly, either. More symptomatic evens were observed in the preparation group, while more radiological events were observed in the loading group. Symptomatic events included blurred vision by either retina ischemia or cerebral infarct in the occipital lobe, mild weakness of the unilateral lower extremity without functional difficulty, and disequilibrium. There were four cases of thrombus formation detected on angiography after completion of coil packing. All thrombi were lyzed after intra-arterial administration of glycoprotein IIb/IIIa inhibitor, and the patency of the involved artery was maintained on the delayed angiography.
A summary of the results of antiplatelet response assays is shown in Table 3. Although the prevalence of aspirin resistance was 6.7%, the prevalence of clopidogrel resistance was remarkably high (56.7%, 73.3%). Every patient with aspirin resistance also showed resistance to clopidogrel. Suboptimal response to aspirin, which can be estimated from ARU ranging between 500 and 550, was found in three patients and two of them also had low responsiveness to clopidogrel.
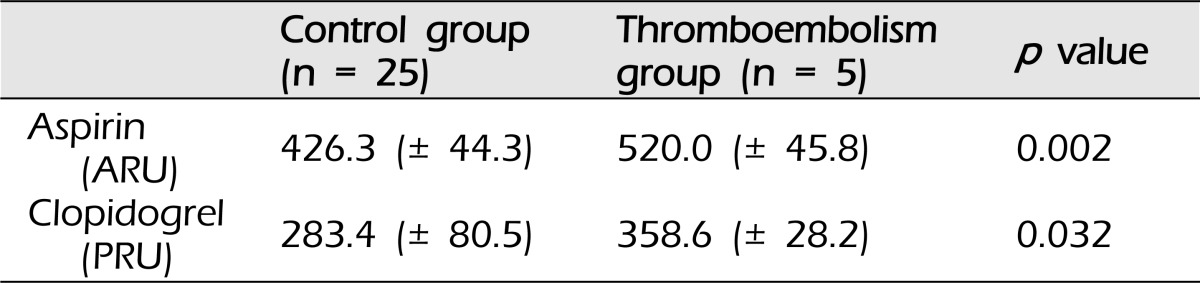
Results of analysis of the relationship between the thromboembolic event and responsiveness to aspirin and clopidogrel are shown in Table 4. Patients who had a thromboembolic event had higher ARU than patients who did not have a thromboembolic event [520.0 (± 45.8) vs. 426.3 (± 44.3)], which was statistically significant (p = 0.002). In addition, a statistically significant difference (p = 0.032) in the results of response assay of clopidogrel was observed between the two groups [358.6 (± 28.2) vs. 283.4 (± 80.5)]. According to the results, suboptimal response to aspirin, including overt resistance, in particular, showed a strong relationship with thromboembolic events.
As a supplement to intraprocedural anticoagulation, which has been almost standardized in all types of coil embolization-related procedures for thromboembolic risk reduction, premedication with antiplatelet agent has at first been adopted to the stent-assisted coil embolization from coronary stenting, considering its beneficial effects.3) As a result of the marked reduction of thromboembolic complications in stent-assisted coil embolization, usage of premedication with an antiplatelet agent has spread to other modes of coil embolization.3) Successive studies have also demonstrated the efficacy of antiplatelet premedication for the multi-catheter technique for coil embolization.7)11) So far, the efficacy of premedication with an antiplatelet agent for balloon-assisted coil embolization has not been well demonstrated. Layton et al. reported a positive reduction of the rate of thromboembolic complication by clopidogrel premedication in a population in which 33% (73 of 221) of patients underwent balloon-assisted coil embolization.13) Since usage of antiplatelet agents has become widespread, there has been a concern about the increment of hemorrhagic complications. However, previous studies have coherently reported that the rate of hemorrhagic event is not altered by antiplatelet premedication.16)27) In short, the benefit of antiplatelet premedication in the conventional coil embolization has not been proved; therefore, since it cannot be fully estimated before the procedure whether conventional coil embolization will be possible and the complication rate of antiplatelet agent premedication is acceptably low, it is reasonable to premedicate the entire population of patients undergoing coil embolization.7)11)
In our institute, due to concerns regarding hemorrhagic events, which can be fatal with high frequency, there was a reluctance to employ antiplatelet agent before the endovascular procedures. We began antiplatelet agent premedication with the stent-assisted coil embolization. We only employed antiplatelet agent for stent-assisted procedures and aspirin with clopidogrel was given orally for three days before the procedure, referring to the guideline report of stent-assisted coil embolization.1) Individual variability of responsiveness to antiplatelet agents has been reported and the duration of antiplatelet premedication for stent-assisted coil embolization has been extended to five days with carefulness concerning about the hemorrhagic risk.4)20)25) After going through serial thromboembolic events and perceiving the low risk of hemorrhage related to antiplatelet agents, we began use of antiplatelet agent for other modes of coil embolization, which include the conventional method, multiple catheter technique, and balloon-assisted procedures.7)11)13) The length of time during which platelet function is compromised can be recognized as a cumulative risk for hemorrhagic event in patients with intracranial aneurysm. In order to minimize the duration of exposing patients to compromised platelet aggregation, we first employed antiplatelet agents using the loading method. Then, resistance to antiplatelet agents became an issue; therefore, we changed our policy of antiplatelet agent premedication to the preparation method, which was adopted from that of stent-assisted coil embolization. In this way, using the drug response assays, we were able to screen out patients with resistance to antiplatelet agents, and deal with the risk.
Antiplatelet effect of aspirin and clopidogrel loading has been established in in vivo laboratory studies. A sufficient antiplatelet effect was achieved 12 hours after loading of aspirin 200 mg.17) A single dose of clopidogrel between 300 mg and 400 mg inhibited platelet aggregations at the same level as the steady state of daily medication by two hours after loading.22) However, little has been reported in relation to comparison of clinical results from use of the methods of antiplatelet agent therapy initiation, and, to the best of our knowledge, no study comparing the effect of high-dose loading of antiplatelet agents and the effect of repeated dosing for the steady state has been reported in the literature on coil embolization.
We compared clinical results of antiplatelet agent premedication according to the rate of periprocedural thromboembolic events. No statistically significant difference was observed between the loading group and the preparation group. This result is consistent with findings of previous in vivo laboratory studies.17)22)24) Although no statistical difference was observed between the two groups, the proportion of conventional coil embolization in the loading group was more than double that in the preparation group. Considering this subtle heterogeneity, there is a possibility that the preparation method may have more efficacies since the double-catheter technique and balloon-assisted technique are regarded as being prone to end in thromboembolism. Further studies comparing the methods of premedication with a larger population are called for.
Antiplatelet agent resistance is a known risk factor for thromboembolic complication.4)22)23) In this study, the he prevalence of aspirin resistance was analogous to the prevalence found in previous reports.4)22)23) On the other hand, the prevalence of clopidogrel resistance was very high and was above the range of prevalence that has been reported in the literature. Poor compliance or underdosing can be inferred as the explanation for this result. It should be emphasized that the duration of premedication could have been inadequate. According to previous reports, clopidogrel requires three to seven days to achieve the steady state for antiplatelet action.4)22) Due to concerns about the hemorrhagic risk, we prescribed only five days of clopidogrel; this could be the reason for higher prevalence of clopidogrel resistance. Although aspirin resistance was relatively low, compared with the previous reports, aspirin also requires four to seven days to achieve the steady state by daily dose.2)4) Although not detected for either suboptimal response or overt resistance, some patients may have had a lesser response to aspirin due to the shortage of dosing duration. What can be drawn from these findings is that prescribing seven days of aspirin and clopidogrel for premedication in the context of the prevention of thromboembolic events seems to be proper since the drug action will be optimal for more patients in this way. Nevertheless, this inherently increases the risk of hemorrhagic events as much as lengthened time of premedication. More evidence supporting the extended duration of antiplatelet agent premedication is required before making a recommendation.
We observed a tendency of low responsiveness to both aspirin and clopidogrel in patients who had thromboembolic events. This is consistent with previous reports addressing the risk of antiplatelet resistance.4)11)24)25) According to the results of the aspirin response assay and analysis with aspects to thromboembolic risk, not only overt resistance to the drug but also suboptimal response to the drug was reported as a significant risk factor for the thromboembolic event described in our study. However, because all of the patients with aspirin resistance and two of three patients with suboptimal response to aspirin had concurrent clopidogrel resistance, we cannot conclude that suboptimal response and resistance to aspirin alone is a strong risk factor. It shall be proper to conclude that co-existing clopidogrel resistance and suboptimal response to aspirin, including overt resistance, is a strong risk factor for thromboembolic event.
Several methods for management of antiplatelet resistance have been proposed; 1) addition of antiplatelet agents of alternative molecular target, 2) using a higher dose of aspirin and clopidogrel, 3) drug for the same molecular target but metabolized by other pathways and 4) active-form drug.25) Some studies have reported that use of a higher dose of either aspirin or clopidogrel reduces the prevalence of antiplatelet agent resistance.4)20)25) They have also concordantly reported that the hemorrhagic risk did not differ between the regular dosing group and the higher dosing group. When the high risk of thromboembolic event due to drug resistance is expected, such as concurrent resistance to both aspirin and clopidogrel, it is practical to give a higher dose of aspirin, clopidogrel, or both. The optimal dosage of aspirin and clopidogrel in cases of drug resistance has not been studied in the population of patients undergoing coil embolization. According to previous studies on clopidogrel, patients loaded with 600 mg showed less prevalence of drug resistance than patients loaded with 300 mg, while the higher doses were not more effective and the maintenance dose of 150 mg brought about less frequent drug resistance than maintaining 75 mg.4)20) For aspirin, the effectiveness increased until the maintenance dosage reached 325 mg, while the higher dose was not more effective.2)4) To sum up, doubling the dosage of antiplatelet agent can be considered against drug resistance. When it comes to premedication for elective coil embolization, this doubling of dosage can be actualized by giving the loading dose additionally before the procedure since the time between the point of detection of drug resistance and the schedule for coil embolization is usually short, one or two days.
To date, the first solution mentioned above has had the most robust background; triple therapy with triflusal or cilostazol showed superb clinical results, compared with dual antiplatelet therapy with aspirin and clopidogrel.9)10)23) In addition, when used as a triple therapy, these two drugs did not increase hemorrhagic risk.9)10)23) Cilostazol showed an even better outcome than a higher dose of clopidogrel.9)10) Both triflusal and cilostazol showed efficacy in prevention of ischemic stroke.15)18) They have also shown efficacy by loading dosage.8)14) These make cilostazol and triflusal suitable for coping with aspirin and/or clopidogrel resistance that appear in patients scheduled for any mode of coil embolization. Nonetheless, because the hemorrhagic risk of triple antiplatelet agent usage has not been elucidated in the context of coil embolization, for now, we cannot make any succinct recommendations. One can try adding cilostazol or triflusal when the risk of thromboembolic event is substantial since reports on coronary stenting have advocated the use of triple antiplatelet agents with addition of one of these two drugs. In our study, there were 17 patients whose resistance to aspirin, clopidogrel, or both was detected by using the VerifyNow® system. After confirming the resistance, we prescribed the loading dosage of either cilostazol or triflusal; ten patients with cilostazol and seven patients with triflusal. Although there was one case of mild gingival bleeding, due a to small number of cases, we did not deduce any statistically meaningful information. Conduct of further studies of the efficacy in thromboembolic risk reduction and increment of hemorrhagic risk of triple antiplatelet agent therapy using cilostazol or triflusal in a larger population is needed.
Our study encompassed a small population with low incidence of the event. Power of statistical testing is an inevitable shortcoming of this study. Based on analysis of this study, repeated study with a larger population may demonstrate superiority of the preparation method. Some records on hemorrhagic events, including groin hematoma, gingival bleeding, or bruises were omitted; therefore, we did not evaluate hemorrhagic events. This is another weakness of this study. Further study should cover periprocedural hemorrhagic events, especially those associated with the extended administration and the higher dosage of aspirin and clopidogrel, and triple antiplatelet therapy with addition of cilostazol or triflusal. Finally, because we did not obtain postoperative MRI in all patients due to the problem of insurance coverage, our study had limitation in over determining radiologic thromboembolic events. A few radiological thromboembolic events may have gone undetected.
The method used for premedication with an antiplatelet agent does not affect the clinical result in terms of the rate of thromboembolic events. Nevertheless, considering the high prevalence of resistance to aspirin and clopidogrel, it is still reasonable to premedicate with antiplatelet agents in the preparation method for drug response assays. Treatment of patients who exhibit low responsiveness to both aspirin and clopidogrel (i.e. concurrent drug resistance) is important, as the thromboembolic risk is high in this group. Use of a higher dose of aspirin and clopidogrel or addition of an alternative drug (cilostazol or triflusal) can be applied against antiplatelet agent resistance. However, because the hemorrhagic risk associated with this supplementary use of antiplatelet agent has not been well-documented, the hemorrhagic risk and the preventive benefit must be weighed.
References
1. Benitez RP, Silva MT, Klem J, Veznedaroglu E, Rosenwasser RH. Endovascular occlusion of wide-necked aneurysms with a new intracranial microstent (Neuroform) and detachable coils. Neurosurgery. 2004; 6. 54(6):1359–1367. discussion 1368. PMID: 15157292.

2. Coleman JL, Alberts MJ. Effect of aspirin dose, preparation, and withdrawal on platelet response in normal volunteers. Am J Cardiol. 2006; 9. 98(6):838–841. PMID: 16950199.

3. Fiorella D, Thiabolt L, Albuquerque FC, Deshmukh VR, McDougall CG, Rasmussen PA. Antiplatelet therapy in neuroendovascular therapeutics. Neurosurg Clin N Am. 2005; 7. 16(3):517–540. PMID: 15990042.

4. Fiorella D. Anti-thrombotic medications for the neurointerventionist: aspirin and clopidogrel. J Neurointerv Surg. 2010; 3. 2(1):44–49. PMID: 21990558.

5. Gasparyan AY, Watson T, Lip GY. The role of aspirin in cardiovascular prevention: implications of aspirin resistance. J Am Coll Cardiol. 2008; 5. 51(19):1829–1843. PMID: 18466797.
6. Gum PA, Kottke-Marchant K, Poggio ED, Gurm H, Welsh PA, Brooks L, et al. Profile and prevalence of aspirin resistance in patients with cardiovascular disease. Am J Cardiol. 2001; 8. 88(3):230–235. PMID: 11472699.

7. Hwang G, Jung C, Park SQ, Kang HS, Lee SH, Oh CW, et al. Thromboembolic complications of elective coil embolization of unruptured aneurysms: the effect of oral antiplatelet preparation on periprocedural thromboembolic complication. Neurosurgery. 2010; 9. 67(3):743–748. discussion 748. PMID: 20651627.
8. Jin EZ, Yu LH, Li XQ. Loading effect of 200 mg cilostazol on platelet inhibition in patients undergoing percutaneous coronary intervention. Int Heart J. 2012; 53(1):1–4. PMID: 22398668.

9. Jeong YH, Lee SW, Choi BR, Kim IS, Seo MK, Kwak CH, et al. Randomized comparison of adjunctive cilostazol versus high maintenance dose clopidogrel in patients with high post-treatment platelet reactivity: results of the ACCEL-RESISTANCE (Adjunctive Cilostazol Versus High Maintenance Dose Clopidogrel in Patients With Clopidogrel Resistance) randomized study. J Am Coll Cardiol. 2009; 3. 53(13):1101–1109. PMID: 19324253.
10. Jeong YH, Hwang JY, Kim IS, Park Y, Hwang SJ, Lee SW, et al. Adding cilostazol to dual antiplatelet therapy achieves greater platelet inhibition than high maintenance dose clopidogrel in patients with acute myocardial infarction: Results of the adjunctive cilostazol versus high maintenance dose clopidogrel in patients with AMI (ACCEL-AMI) study. Circ Cardiovasc Interv. 2010; 2. 3(1):17–26. PMID: 20118150.
11. Kang HS, Kwon BJ, Kim JE, Han MH. Preinterventional clopidogrel response variability for coil embolization of intracranial aneurysms: clinical implications. AJNR Am J Neuroradiol. 2010; 8. 31(7):1206–1210. PMID: 20223886.

12. Kim HK, Hwang SK, Kim SH. Types of thromboembolic complications in coil embolization for intracerebral aneurysms and management. J Korean Neurosurg Soc. 2009; 9. 46(3):226–231. PMID: 19844623.

13. Layton KF, Cloft HJ, Gray LA, Lewis DA, Kallmes DF. Balloon-assisted coiling of intracranial aneurysms: evaluation of local thrombus formation and symptomatic thromboembolic complications. AJNR Am J Neuroradiol. 2007; Jun-Jul. 28(6):1172–1175. PMID: 17569982.

14. Lee HW, Lim MS, Seong SJ, Lee J, Park J, Seo JJ, et al. A phase I study to characterize the multiple-dose pharmacokinetics, pharmacodynamics and safety of new enteric-coated triflusal formulations in healthy male volunteers. Expert Opin Drug Metab Toxicol. 2011; 12. 7(12):1471–1479. PMID: 22098139.

15. Matsumoto M. Cilostazol in secondary prevention of stroke: impact of the Cilostazol Stroke Prevention Study. Atheroscler Suppl. 2005; 12. 6(4):33–40. PMID: 16275125.

16. Matsumoto Y, Kondo R, Matsumori Y, Shimizu H, Takahashi A, Tominaga T. Antiplatelet therapy for prevention of thromboembolic complications associated with coil embolization of unruptured cerebral aneurysms. Drugs R D. 2012; 3. 12(1):1–7. PMID: 22242721.

17. Meves SH, Neubauer H, Overbeck U, Endres HG. Is there an ideal way to initiate antiplatelet therapy with aspirin? A crossover study on healthy volunteers evaluating different dosing schemes with whole blood aggregometry. BMC Res Notes. 2011; 4. 4:106. PMID: 21466682.

18. Murdoch D, Plosker GL. Triflusal: a review of its use in cerebral infarction and myocardial infarction, and as thromboprophylaxis in atrial fibrillation. Drugs. 2006; 66(5):671–692. PMID: 16620146.
19. Nielsen HL, Kristensen SD, Thygesen SS, Mortensen J, Pedersen SB, Grove EL, et al. Aspirin response evaluated by the VerifyNow Aspirin System and light transmission aggregometry. Thromb Res. 2008; 123(2):267–273. PMID: 18499236.

20. Patti G, Colonna G, Pasceri V, Pepe LL, Montinaro A, Di Sciascio G. Randomized trial of high loading dose of clopidogrel for reduction of periprocedural myocardial infarction in patients undergoing coronary intervention: results from the ARMYDA-2 (Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty) study. Circulation. 2005; 4. 111(16):2099–2106. PMID: 15750189.

21. Ryu DS, Hong CK, Sim YS, Kim CH, Jung JY, Joo JY. Anti-platelet drug resistance in the prediction of thromboembolic complications after neurointervention. J Korean Neurosurg Soc. 2010; 10. 48(4):319–324. PMID: 21113358.

22. Savcic M, Hauert J, Bachmann F, Wyld PJ, Geudelin B, Cariou R. Clopidogrel loading dose regimens: kinetic profile of pharmacodynamic response in healthy subjects. Semin Thromb Hemost. 1999; 25(Suppl 2):15–19. PMID: 10440417.
23. Suh JW, Kim SY, Park JS, Kim YS, Kang HJ, Koo BK, et al. Comparison of triple antiplatelet therapy including triflusal and conventional dual therapy in patients who underwent drug-eluting stent implantation. Int Heart J. 2009; 11. 50(6):701–709. PMID: 19952467.

24. Tourmousoglou CE, Rokkas CK. Clopidogrel and aspirin in cardiovascular medicine: responders or not--current best available evidence. Cardiovasc Hematol Agents Med Chem. 2008; 10. 6(4):312–322. PMID: 18855644.
25. Uchiyama S. Clopidogrel resistance: identifying and overcoming a barrier to effective antiplatelet treatment. Cardiovasc Ther. 2011; 12. 29(6):e100–e111. PMID: 21883990.

26. van Rooij WJ, Sluzewski M. Procedural morbidity and mortality of elective coil treatment of unruptured intracranial aneurysms. AJNR Am J Neuroradiol. 2006; 9. 27(8):1678–1680. PMID: 16971613.
27. Workman MJ, Cloft HJ, Tong FC, Dion JE, Jensen ME, Marx WF, et al. Thrombus formation at the neck of cerebral aneurysms during treatment with Guglielmi detachable coils. AJNR Am J Neuroradiol. 2002; 10. 23(9):1568–1576. PMID: 12372750.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download