Abstract
Osseointegrated implants are now commonplace in contemporary dentistry. However, a number of complications can occur around dental implants, including peri-implantitis, maxillary sinusitis, osteomyelitis, and neoplasms. There have been several reports of a malignant neoplasm occurring adjacent to a dental implant. In this report, we describe 2 such cases. One case was that of a 75-year-old man with no previous history of malignant disease who developed a solitary plasmacytoma around a dental implant in the left posterior mandible, and the other was that of a 43-year-old man who was diagnosed with squamous cell carcinoma adjacent to a dental implant in the right posterior mandible. Our experiences with these 2 cases suggest the possibility of a relationship between implant treatment and an inflammatory cofactor that might increase the risk of development of a malignant neoplasm.
Dental implants and osseointegration are now common issues in daily dental practice.1 Dental implants play a substantial role in modern dentistry, as the use of oral implants is a highly successful and widely accepted treatment option. The general success rates are >90%, and the overall frequency of implant loss is low. However, despite these high success and survival rates, there has been an increasing number of reports concerning the complications associated with their use. A systemic review of the literature over decades found that inflammatory diseases, such as peri-implantitis, maxillary sinusitis, and osteomyelitis, are common around implants and account for a large percentage of complications.2 McDermott et al.3 reported that the overall frequency of implant complications after the placement of dental implants was 13.9% (94 of 677), and that 10.2% (69 of 677) of these complications involved inflammation. Slightly over half of the complications (52.2%, 36 of 69) were major, involving more than 2 inflammatory complications or implant failure. Most inflammatory complications in that study were attributed to implant mobility (4.0%, 27 of 677), infection (2.4%, 16 of 677), or pain (1.6%, 11 of 677). Most of the common complications can be managed relatively easily by prescribing antibiotics or removing the implant.
However, unusual severe complications can occur around the implant in the absence of a specific identifiable cause. In recent years, there have been several reports of malignant tumors developing in close proximity to dental implants. Thus far, the development of a malignant tumor in the vicinity of an osseointegrated dental implant has been an exceedingly rare event. Therefore, it is not known whether implants are the direct cause of such tumors or which etiologic mechanisms might be involved. Herein, we report the cases of 2 patients who developed a malignant neoplasm in close proximity to a dental implant, discuss the diagnostic process, and speculate on the etiology of these tumors.
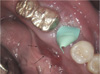
A 75-year-old man was referred to Kyung-Hee Dental Hospital from a local clinic for evaluation of paresthesia of the left lower lip and a firm mass on the left buccal side. He had a slight swelling localized in the area of the body of the left mandible. On palpation, the swelling was hard, attached to the body of the mandible, and slightly tender. Paresthesia was present on the left lower lip. An intraoral examination revealed a large erythematous mass in the lingual vestibular area (Fig. 1). The regional lymph nodes could not be detected by palpation. His medical history was unremarkable. According to his dental history, the patient had experienced dull pain in his left lower molar region 7 months earlier and underwent extraction of the left lower first molars because of a periapical abscess (Fig. 2A). The patient underwent implant surgery approximately 4 months after the tooth extraction (Fig. 2B). Two months later, explantation for peri-implantitis was performed. Since then, he had experienced continuous dull pain and swelling in the body of the left mandible and reported numbness of the left lower lip. The panoramic radiograph showed ill-defined, permeative bone destruction from the left lower premolar area to the second molar area. Comparison of this panoramic image with the one obtained from the local clinic on his first visit (Fig. 2A) showed that the lesion had expanded into the mandibular canal (Fig. 3A). On the periapical view, there was infiltrative bone destruction and an enlarged soft tissue shadow (Fig. 3B). Cone-beam computed tomography was performed to obtain more detailed information about the lesions. The sagittal images showed osteolytic destruction in the left mandibular body area and loss of cortication in the mandibular canal (Fig. 4A). On the cross-sectional view, there was partial perforation and erosion of the buccal and lingual cortical plates (Fig. 4B). An initial biopsy was performed under local anesthesia. A histopathologic examination revealed sheets of atypical plasmacytoid cells (Fig. 5A). On immunohistochemistry, the tumor cells were negative for CD20, a B-cell marker (Fig. 5B), but positive for CD138, a plasma cell marker (Fig. 5C). While the kappa light chain was expressed in all of the tumor cells (Fig. 5D), the lambda light chain was negative, indicating that the tumor cell population was monoclonal. The final diagnosis was solitary plasmacytoma. The patient is currently receiving radiotherapy.
A 43-year-old man presented with a complaint of a painful ulcerative lesion around the area of a dental implant in the right posterior mandible that had first noticed about 7 months earlier. According to his dental history, he underwent implant surgery a year earlier. His medical history was unremarkable. An oral examination revealed an ulcerated gingival swelling on the alveolar ridge around the implant, and he complained of severe pain during palpation. Gingival redness was observed at the site of the lesion. The panoramic radiographic images showed bony destruction, an ill-defined border around the dental implant (Fig. 6A), and an enlarged soft tissue shadow on the periapical view (Fig. 6B). Ultrasonographic images in B-mode indicated a well-demarcated heterogeneous mass (30 mm×15 mm) with localized bone destruction (Fig. 7A). A color Doppler sonogram showed that newly formed blood vessels supplied the interior and periphery of the mass (Fig. 7B). An incisional biopsy showed that the ulceration of the alveolar mucosa was caused by loss of epithelial integrity in response to malignant transformation (Fig. 8A). The dysplastic squamous cells had invaded the subjacent connective tissue and there was a marked infiltration of inflammatory cells. Individual tumor cells exhibited nuclear pleomorphism, prominent nucleoli, and aberrant mitoses (Fig. 8B), with keratin pearls formed in some tumor nests. Based on these clinicoradiographic and histopathologic findings, the final diagnosis was well-differentiated oral squamous cell carcinoma.
A malignant tumor occurred in the bone marrow in one of the patients described here and around the soft tissue in the other. In the first patient, a solitary plasmacytoma (i.e., a malignant proliferation of monoclonal plasma cells), had developed around a dental implant in the posterior mandible. A few reports have described hematopoietic malignant tumors developing in close proximity to dental implants. Poggio4 reported the case of a patient in whom a plasmacytoma developed 3 years after implant surgery at the surgical site and then metastasized to the spine 12 years later. Junquera et al.5 likewise reported the case of a patient with multiple myeloma adjacent to a mandibular dental implant that also involved the cranial calotte and the thoracic and lumbar spine.
It is unknown whether a dental implant can be a direct cause of malignancy and, if so, which mechanisms are involved in the pathogenesis. Few of the interactions between the implant interface and bone marrow cells have been studied. In one study, the relationships between titanium implants in bone and the immune and hematopoietic cell populations in the adjacent bone marrow were investigated in a mouse model using miniaturized implants in the femoral diaphysis. The cells at the bone marrow-titanium interface were directly exposed to ions and particulate matter emitted from the titanium surface. This process caused chronic local irritation, leading to the production of giant cells not normally found in the bone marrow.6 These giant cells may have originated from the fusion of monocytes and macrophages when the foreign material was too large to phagocytose and were associated with an environment of chronic low-level inflammation. It remains to be demonstrated whether these giant cells secrete functionally active cytokines that could affect the hematopoietic system. Previous research has shown that splenic macrophages activated by various foreign substances secrete soluble factors that may disturb the production of lymphocytes in the bone marrow and the balance within the hematopoietic system. This disruption could affect the immune capacity of the host and predispose the host to potentially neoplastic dysregulation of bone marrow cells.789
Trauma to the skeleton can cause the release of cytokines, resulting in proliferation of plasma and stromal cells in bone.10 Stromal cells in particular release interleukin-6, which can support the growth of plasma cells, monoclonal B-lymphocytes, and osteoclasts.11 Thus, dental implants could be regarded as a form of mandibular trauma that produces ongoing inflammation, leading to the overstimulation of plasma cells, which could culminate in a malignant clone, as seen in chronic infectious disease processes.1213
In the second patient, a malignant tumor developed in the soft tissue around the implant. Other cases of oral cancer developing around implants have been reported. Block and Scheufler reported the case of a 72-year-old man with oral squamous cell carcinoma that manifested as peri-implant bone loss in the left mandibular region. That patient had a longstanding history of leukoplakia and verrucous cell carcinoma at different sites and at different times.14 Czerninski et al.15 described 2 cases of oral squamous cell carcinoma: one in a patient with high tobacco consumption and oral lichen planus and the other in a patient with a history of oral cancer and colon cancer. Eguia del Valle et al.16 reported the case of a 76-year-old man with primary oral squamous cell carcinoma arising around a dental implant and mimicking peri-implantitis. The patient did not have a known history of malignant lesions or risk factors.
It is difficult to explain the relationship between dental implants and development of oral squamous cell carcinoma. Some authors have argued that implant placement could lead to the development of oral squamous cell carcinoma arising from the periodontal epithelium because of damage to the periodontal tissue and loss of the periodontal ligament.16 Healthy periodontal tissue acts as a natural barrier to the progression of a malignant tumor, whereas a dental implant provides an environment conducive to rapid growth and infiltration of a malignant lesion into bone.17
The following issues in our 2 patients should be considered. In the first case, the appropriate routine treatment protocol of reattempting endodontic treatment for early periapical lesions was not followed, and the implant was placed immediately after tooth extraction. The healing of the hard and soft tissues around the surgical site should have been evaluated radiographically before implant surgery. Careful observation of the healing of the soft and hard tissues after implant surgery was also necessary in the second case. In particular, the state of the gingiva should have been assessed before embarking on the final restoration, such that an accurate diagnosis and appropriate intervention would have been made in a timely manner. Failure to follow the appropriate protocol meant that the golden time for effective treatment was missed, leading to extensive destruction of the surrounding tissues.
In conclusion, even though a clear cause-effect relationship was not established in these 2 patients, it is possible that implant treatment may represent an inflammatory cofactor that increases the risk of development of a malignant neoplasm. The 2 patients reported here developed mucosal hyperplasia and bone resorption around their dental implants, so it is imperative to carry out an exhaustive differential diagnosis and to perform a subsequent histopathologic examination in order to make a definitive diagnosis as soon as possible. This is particularly important when the lesion is of sudden onset and progresses rapidly without responding to anti-inflammatory therapy. Otherwise, an accurate diagnosis of a malignant neoplasm may be delayed, leading to extensive destruction of the surrounding tissues and a poor prognosis.
Figures and Tables
Fig. 2
A. A cropped panoramic radiograph reveals a periapical radiolucency in the left mandibular molar. B. A cropped panoramic radiograph shows a dental implant that was placed approximately 4 months after tooth extraction.
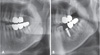
Fig. 3
A. A cropped panoramic radiograph shows ill-defined, permeative bone destruction in the area from the left lower premolar to the second molar. When compared with Figure 2A, the lesion has expanded into the mandibular canal. B. Infiltrative bone destruction and an enlarged soft tissue shadow (arrows) can be seen in the periapical view.
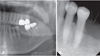
Fig. 4
A. A sagittal cone-beam computed tomographic (CBCT) image shows osteolytic destruction in the area of the left mandibular body and loss of cortication in the mandibular canal. B. Cross-sectional CBCT images demonstrate partial perforation and erosion of the buccal and lingual cortical plates.

Fig. 5
A. A histopathologic exam demonstrates diffuse proliferation of atypical plasmacytoid cells (H&E stain). Immunohistochemical staining revealed that the tumor cells were negative for CD20 (B), but positive for CD138 (C), a plasma cell marker. D. Of the two types of immunoglobulin light chain, only the kappa chain showed immunoreactivity to tumor plasma cells (A–D, original magnification 400×).
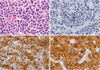
Fig. 6
A. A cropped panoramic radiograph shows bone destruction with an ill-defined border around the dental implants. B. A periapical radiograph shows an enlarged soft tissue shadow.

Fig. 7
A. Ultrasonographic images in B-mode show a well-demarcated heterogeneous mass (30 mm×15 mm) with localized bone destruction. B. Color Doppler sonograms show newly formed blood vessels supplying the interior and periphery of the mass.
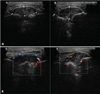
Fig. 8
A. A representative photomicrograph shows invasion of dysplastic squamous cells into the underlying connective tissue (H&E stain, original magnification 40×). B. Tumor nests composed of atypical keratinocytes showing cellular pleomorphism, brisk mitosis, and dyskeratosis (H&E stain, original magnification 400×).

Acknowledgements
The authors express their special thanks to Young-Ah Cho, former Assistant Professor of the Department of Oral and Maxillofacial Pathology, Kyung Hee University Graduate School for providing advice about the histological evaluation and for editing the manuscript.
References
1. Henry PJ. Oral implant restoration for enhanced oral function. Clin Exp Pharmacol Physiol. 2005; 32:123–127.

2. Atieh MA, Alsabeeha NH, Faggion CM Jr, Duncan WJ. The frequency of peri-implant diseases: a systematic review and meta-analysis. J Periodont. 2013; 84:1586–1598.

3. McDermott NE, Chuang SK, Woo VV, Dodson TB. Complications of dental implants: identification, frequency, and associated risk factors. Int J Oral Maxillofac Implants. 2003; 18:848–855.

4. Poggio CE. Plasmacytoma of the mandible associated with a dental implant failure: a clinical report. Clin Oral Implants Res. 2007; 18:540–543.

5. Junquera L, Gallego L, Pelaz A. Multiple myeloma and bisphosphonate-related osteonecrosis of the mandible associated with dental implants. Case Rep Dent. 2011; 2011:568246.

6. Rahal MD, Brånemark PI, Osmond DG. Response of bone marrow to titanium implants: osseointegration and the establishment of a bone marrow-titanium interface in mice. Int J Oral Maxillofac Implants. 1993; 8:573–579.
7. Fulop GM, Osmond DG. Regulation of bone marrow lymphocyte production. III. Increased production of B and non-B lymphocytes after administering systemic antigens. Cell Immunol. 1983; 75:80–90.
8. Pietrangeli CE, Osmond DG. Regulation of B-lymphocyte production in the bone marrow: mediation of the effects of exogenous stimulants by adoptively transferred spleen cells. Cell Immunol. 1987; 107:348–357.

9. Osmond DG, Priddle S, Rico-Vargas S. Proliferation of B cell precursors in bone marrow of pristane-conditioned and malaria-infected mice: implications for B cell oncogenesis. Curr Top Microbiol Immunol. 1990; 166:149–157.

10. Hussein MA, George R, Rybicki L, Karam MA. Skeletal trauma preceding the development of plasma cell dyscrasia: eight case reports and review of the literature. Med Oncol. 2003; 20:349–354.

11. Caligaris-Cappio F, Bergui L, Gregoretti MG, Gaidano G, Gaboli M, Schena M, et al. Role of bone marrow stromal cells in the growth of human multiple myeloma. Blood. 1991; 77:2688–2693.

12. McGrory JE, Pritchard DJ, Unni KK, Ilstrup D, Rowland CM. Malignant lesions arising in chronic osteomyelitis. Clin Orthop Relat Res. 1999; 362:181–189.

13. Oehler R, Weingartmann G, Manhart N, Salzer U, Meissner M, Schlegel W, et al. Polytrauma induces increased expression of pyruvate kinase in neutrophils. Blood. 2000; 95:1086–1092.

14. Block MS, Scheufler E. Squamous cell carcinoma appearing as peri-implant bone loss: a case report. J Oral Maxillofac Surg. 2001; 59:1349–1352.

15. Czerninski R, Kaplan I, Almoznino G, Maly A, Regev E. Oral squamous cell carcinoma around dental implants. Quintessence Int. 2006; 37:707–711.
16. Eguia del Valle A, Martinez-Conde Llamosas R, López Vicente J, Uribarri Etxebarria A, Aguirre Urizar JM. Primary oral squamous cell carcinoma arising around dental osseointegrated implants mimicking peri-implantitis. Med Oral Patol Oral Cir Bucal. 2008; 13:E489–E491.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download