Abstract
Purpose
Underlying bone sclerosis is frequently observed in clinical settings when oral squamous cell carcinoma (OSCC) invades the jaw bone. The aim of this study was to assess the prevalence and characteristics of underlying bone sclerosis in patients with OSCC.
Materials and Methods
We retrospectively reviewed the computed tomographic (CT) images of 131 patients who underwent mandibulectomy between January 2012 and December 2015 to treat OSCC. The presence, degree, and extent of underlying bone sclerosis were assessed on CT images and correlated with the following imaging patterns of bone invasion: cortical invasion, medullary invasion with a smooth margin, and medullary invasion with an irregular margin. The chi-square test was used to determine the relationships between the variables.
Results
The prevalence of underlying bone sclerosis on CT images was 70.1% (47 of 67). The prevalence was 85.7% (42 of 49) in patients with medullary invasion, but it was 27.8% (5 of 18) in patients with only cortical invasion, indicating a significant increase in the prevalence of underlying bone sclerosis in patients with medullary invasion (P<.05). Aggressive patterns of bone invasion were associated with increases in the degree and extent of the underlying bone sclerosis (P<.05).
Oral squamous cell carcinoma (OSCC) frequently invades the adjacent jaw bone in about 12% to 56% of cases.123 It is important to determine the appropriate margin of surgical resection by precisely analyzing the extent of bone invasion with preoperative computed tomographic (CT) images.4
Sclerosis in the remaining margin of the alveolar bone has been considered a primary means to differentiate inflammatory bone loss due to periodontal disease from bone invasion by gingival cancer.5 However, underlying bone sclerosis is frequently observed in clinical settings when OSCC invades the jaw bone. As a result, the extent of the tumor invasion is difficult to determine and is often overestimated by radiologists.
There have been few studies regarding sclerosis adjacent to a tumor mass. One study revealed that nearly half of nasopharyngeal carcinoma patients had developed sclerosis in the pterygoid process adjacent to a tumor mass.6 Another study showed that the presence of sclerosis in the cricoid and arytenoid cartilage might be an early indication of microscopic disease and should increase the suspicion of cartilaginous invasion by laryngeal carcinoma.7
Recently, it has become increasingly evident that the microenvironment surrounding a tumor plays a significant role in the progression of OSCC.8 A better understanding of the underlying bone change will increase our knowledge of the mechanism of bone invasion and hopefully lead to the development of minimally invasive surgical procedures.
The aim of this study was to assess the prevalence and characteristics of underlying bone sclerosis in patients with OSCC.
This retrospective study was approved by the Institutional Review Board of Seoul National Dental Hospital (IRB007/01-15).
Between January 2012 and December 2015, 131 patients who underwent mandibulectomy to treat OSCC that was confirmed by histopathology to have invaded bone were enrolled. Subjects with recurrence, a lesion in any area other than the gingiva (e.g., the cheek, lip, tongue, mouth floor, or pharynx), or who received preoperative radiotherapy or chemotherapy were excluded. In total, 81 subjects were included in the final analysis, including 56 males and 25 females. The mean age was 64.6 years.
All patients underwent a contrast-enhanced CT scan; multiplanar CT images were reconstructed with a contiguous 2- to 3-mm slice thickness and interval. The CT images were evaluated through consensus by 2 experienced oral and maxillofacial radiologists in a picture archiving and communication system (Infinitt PACS, Infinitt Healthcare, Seoul, Korea).
Underlying bone was defined as the region directly beneath the deepest bone destroyed by OSCC. The presence, degree, and extent of underlying bone sclerosis, defined as an increased attenuation of the medullary cavity, were assessed on the CT images and correlated with the imaging patterns of bone invasion.
The degree of sclerosis in the underlying bone was assessed as being either absent, subtle, or prominent in comparison with the contralateral side of the mandible. The extent of sclerosis was recorded as follows: 0, no sclerosis; 1, sclerosis was present but did not extend over the mandibular canal; 2, sclerosis was present and extended over the mandibular canal.
The imaging pattern of the mandibular invasion was also evaluated and categorized as either cortical invasion, medullary invasion with a smooth margin, or medullary invasion with an irregular margin. A smooth margin was defined as a well-defined border with a narrow transition zone and no residual bone behind the border. An irregular margin was defined as an ill-defined border with a wide transition zone and a finger-like extension into the surrounding bone.9
Chi-square tests were used to determine the relationship between the variables. A P value of <.05 was considered statistically significant. All statistical analyses were performed using SPSS version 22 (IBM Corporation, Armonk, NY, USA).
Of the 81 cases in this study, 14 had bone destruction up to the inferior cortex of the mandible. It was not possible to further analyze these 14 cases because no underlying bone remained.
The prevalence of underlying bone sclerosis on CT images was 70.1% (47 of 67). The prevalence was 85.7% (42 of 49) in patients with medullary invasion, but was 27.8% (5 of 18) in patients with only cortical invasion, indicating a significant increase in the prevalence of underlying bone sclerosis in patients with medullary invasion (P<.05).
Aggressive patterns of bone invasion were associated with increases in the degree and extent of the underlying bone sclerosis (P<.05) (Tables 1 and 2).
Of the 18 cases in the cortical invasion group, 13 had no underlying bone sclerosis. Subtle sclerosis that was limited to the alveolar bone was observed in the remaining 5 cases (Fig. 1).
Of the 16 cases in the medullary invasion with a smooth margin group, 11 had underlying bone sclerosis. There were 6 cases of subtle sclerosis that was limited to the alveolar bone, 2 cases of subtle sclerosis that included the basal bone, and 3 cases of prominent sclerosis that was limited to the alveolar bone. None of the cases in this group had prominent sclerosis that included the basal bone.
Of the 33 cases in the medullary invasion with an irregular margin group, 31 had underlying bone sclerosis. There were 13 cases of subtle sclerosis that was limited to the alveolar bone, 5 cases of subtle sclerosis that included the basal bone, 4 cases of prominent sclerosis that was limited to the alveolar bone, and 9 cases of prominent sclerosis that included the basal bone.
Alveolar bone loss from OSCC may appear very similar to periodontal disease.9 Reactive sclerosis in surrounding bone is usually observed in inflammatory lesions such as periodontitis. Previous studies have demonstrated that, due to rapid bone destruction, reactive sclerosis around malignant lesions, including OSCC, is not visible on conventional radiographs. Therefore, the presence of sclerosis adjacent to an osteolytic lesion is considered an important imaging finding that distinguishes periodontitis from OSCC.5
However, in the current study, underlying bone sclerosis was found on as many as 70% of the CT images. In such cases, the underlying sclerosis can make it difficult to distinguish inflammatory disease from a malignant lesion. Compared to plain radiographs, CT images can show subtle trabecular structures in 3 dimensions and detect changes in the attenuation of the medullary space.10 As a result, there may be a discrepancy between the prevalence of underlying sclerosis determined from CT images and plain radiographs. For the majority of subtle sclerosis cases, it may be difficult to observe a significant underlying bone change on panoramic radiographs.
When analyzing preoperative CT images of OSCC patients, it is important to precisely evaluate the extent of bone involvement. However, the presence of underlying bone sclerosis adjacent to the tumor mass can make it difficult for radiologists to determine the margin of surgical resection, because the sclerosis may have been caused by tumor cells that invaded the underlying bone or by a reactive change not involving tumor cells.
OSCC bone invasion is not a simple bone resorption by osteoclasts alone. Rather, it is a complicated process involving numerous fibroblasts, osteoblasts, and cytokines.11 Tumor cells produce several cytokines, such as interleukin-6 and the parathyroid hormone-related protein (PTHrP). It induces fibroblasts and osteoblasts to synthesize receptor activator of NF-κB ligand (RANKL), and it subsequently induces osteoclast formation.12 In addition, osteoclasts release various growth factors, such as fibroblast growth factor, during bone destruction.13 This mechanism suggests that osteoblasts and fibroblasts can change in response to tumor cell stimulation. Therefore, underlying bone sclerosis may be the result of reactive bone change rather than tumor cell infiltration.
Further research is needed to examine changes to the underlying bone marrow using magnetic resonance imaging and positron emission tomography-CT images and to analyze these changes according to histopathologic correlations.
In the current study, more than two-thirds of OSCC cases with bone involvement showed underlying bone sclerosis. On CT images, reactive sclerosis in the remaining margin of the jaw bone should not be used as a primary means to differentiate periodontal inflammatory lesions from those resulting from OSCC.
Figures and Tables
 | Fig. 1Coronal computed tomographic images using the bone window setting (A) and soft-tissue window setting (B) showing subtle underlying bone sclerosis that is confined to the alveolar bone portion (arrow) and has a cortical invasion pattern. |
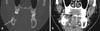 | Fig. 2Coronal computed tomographic images using the bone window setting (A) and soft-tissue window setting (B) showing prominent underlying bone sclerosis that is confined to the alveolar bone portion (arrow) and has a smooth medullary invasion pattern. |
References
1. Hoffmannová J, Foltán R, Vlk M, Sipos M, Horká E, Pavliková G, et al. Hemimandibulectomy and therapeutic neck dissection with radiotherapy in the treatment of oral squamous cell carcinoma involving mandible: a critical review of treatment protocol in the years 1994-2004. Int J Oral Maxillofac Surg. 2010; 39:561–567.

2. Lubek J, El-Hakim M, Salama AR, Liu X, Ord RA. Gingival carcinoma: retrospective analysis of 72 patients and indications for elective neck dissection. Br J Oral Maxillofac Surg. 2011; 49:182–185.

3. Chen YL, Kuo SW, Fang KH, Hao SP. Prognostic impact of marginal mandibulectomy in the presence of superficial bone invasion and the nononcologic outcome. Head Neck. 2011; 33:708–713.

4. Uribe S, Rojas LA, Rosas CF. Accuracy of imaging methods for detection of bone tissue invasion in patients with oral squamous cell carcinoma. Dentomaxillofac Radiol. 2013; 42:20120346.

5. Koenig LJ. Diagnostic imaging. Oral and maxillofacial. Salt Lake City: Amirsys Pub;2012. p. 2–28.
6. Shatzkes DR, Meltzer DE, Lee JA, Babb JS, Sanfilippo NJ, Holliday RA. Sclerosis of the pterygoid process in untreated patients with nasopharyngeal carcinoma. Radiology. 2006; 239:181–186.

7. Munoz A, Ramos A, Ferrando J, Gomez B, Escudero L, Relea F, et al. Laryngeal cacinoma: sclerotic apperance of the cricoid and arytenoid cartilage-CT-pathologic correlation. Radilogy. 1993; 189:433–437.
9. White SC, Pharoah MJ. Oral radiology: principles and interpretation. 7th ed. St. Louis: Elsevier;2014. p. 430.
10. Nakayama E, Yoshiura K, Yuasa K, Kanda S, Saitoh M, Kage W, et al. A study of the association between the prognosis of carcinoma of the mandibular gingiva and the pattern of bone destruction on computed tomography. Dentomaxillofac Radiol. 2000; 29:163–169.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download