Abstract
Squamous cell carcinoma (SCC) arising within the lining of an odontogenic keratocyst (OKC) is a rare occurrence. Although potentially locally destructive, OKC is a benign odontogenic process that typically presents with clinical and radiographic features characteristic of a benign intraosseous neoplasm. We present the clinical and radiographic features of a maxillary mass that demonstrated SCC arising from the lining of an OKC. Although the initial clinical and radiographic presentation suggested an infection or malignant neoplasm, biopsies revealed an infiltrative well-differentiated SCC contiguous with and arising from the focus of a pre-existing OKC. The patient subsequently underwent a type II hemi-maxillectomy with neoadjuvant chemoradiation. This report discusses the clinical and radiographic features associated with intraosseous malignancies, especially those arising from an otherwise benign odontogenic lesion. While the majority of OKCs are benign, the current report illustrates the potential for carcinomatous transformation within the lining of an OKC.
Odontogenic keratocyst (OKC) is a benign cystic tumor of odontogenic origin. It is considered a clinically significant diagnosis owing to its locally destructive behavior and the 0%-62% recurrence rates reported following treatment.1 While the majority of OKCs are sporadic, multiple OKCs are seen in patients with the inherited genetic condition nevoid basal cell carcinoma syndrome. When categorized as a benign odontogenic tumor, OKCs comprise 11% of jaw tumors, with a peak incidence in the second and third decades of life.234 OKC is typically reported to occur in the posterior mandible, and is associated with unerupted/impacted teeth (25%-40%).56 Patients typically present with slowly expansile, painless masses of the jaws that are often associated with unerupted/impacted teeth. The radiographic presentation of OKC can mimic that of a range of benign intraosseous neoplasms, both odontogenic and non-odontogenic; they present as unilocular or multilocular radiolucencies with well-demarcated, smooth or scalloped, and corticated borders, and are often associated with an impacted/unerupted or displaced tooth within the posterior mandible. Common findings include local destruction, including root resorption, tooth displacement, and expansion.
Large maxillary OKCs have the potential to become secondarily infected, and tend to be more locally destructive owing to secondary infection and the structural nature of the maxillary bone. Malignant transformation of the lining of OKCs is exceedingly rare.47891011 In this report, we describe a case of SCC arising from the epithelial lining of a maxillary OKC. We include the associated radiographic presentation and histopathological features, and review the literature relevant to this uncommon occurrence.
A 43-year-old woman was referred to an oral surgeon with pain and swelling in the right maxillary canine-premolar region of several weeks duration. The patient was initially aware of a painless swelling that had become progressively painful over the last month. The patient was otherwise well and in generally good health. She reported no constitutional signs or symptoms. An extraoral examination revealed tender, fluctuant swelling that extended from the right nasolabial region to the right infraorbital region. The skin overlying the mass was unremarkable except for minimal erythema. Intraoral examination revealed a diffuse buccal swelling in the region of the missing right maxillary canine. The mucosa overlying the area was intact, with no evidence of fistulous tracts or ulceration. The teeth in the right maxillary quadrant were vital on electric pulp testing. There was no evidence or report of anesthesia, paresthesia, visual disturbance, or nasal obstruction. There was no evidence of tooth mobility or tenderness.
A panoramic radiograph revealed an impacted right maxillary canine with a pericoronal radiolucency. Intimately associated with its apical region and involving much of the right maxillary sinus floor and cavity, there was a large, ill-defined, destructive radiolucency. The radiolucency appeared to ‘blow out’ much of the recognizable antral region, with poorly defined borders extending superiorly to involve the orbital floor region. The exam also revealed a few edentulous spaces, maxillary midline diastema, and generalized mild alveolar crestal bone loss. (Fig. 1).
Based on the radiographic presentation and the amount of destruction noted on the panoramic image, computed tomography (CT) was performed to further evaluate the region of interest. Axial and coronal CT sections revealed an ill-defined lytic mass with soft-tissue density destroying and displacing much of the roof and lateral wall of the right maxillary sinus, obliterating the entire antral cavity. Erosion of the orbital floor and the right lateral nasal wall was noted, but the right pterygoid plate appeared intact. An impacted right maxillary canine was noted, associated with a well-demarcated pericoronal radiolucency (Fig. 2). Given the complex and destructive radiographic findings, the working differential diagnosis of a malignancy was considered but the possibility of an aggressive infection was also considered.
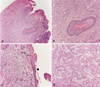
A representative biopsy of the area was obtained. Histopathological examination revealed multiple intraosseous soft tissue fragments, the bulk of which were composed of a well-differentiated squamous cell carcinoma. Of note, in a few foci there were strips of cystic epithelium. The cyst lining demonstrated findings pathognomonic of OKC. The lining was uniform in thickness with basal cell hyperchromasia and regimentation. Surface corrugation and parakeratinization were appreciable. The cyst lining appeared atypical and frankly dysplastic in foci. Contiguous with and arising from within these dysplastic cystic foci, there were numerous infiltrative nests of neoplastic epithelium consistent with SCC. Lymphovascular and perineural invasion were not observed. Fragments of inflamed antral mucosa were also noted (Fig. 3). These findings were diagnostic of an SCC contiguous with and arising from the epithelial lining of an OKC.
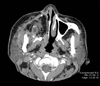
The patient subsequently underwent a type II hemimaxillectomy with neoadjuvant chemoradiation. An obturator and a radial forearm free flap were used to reconstruct the surgical defect. The postoperative radiographic changes are demonstrated in Figure 4.
OKC is a benign, clinically significant cystic tumor of odontogenic origin. The large majority of OKCs are sporadic and non-syndromic. Despite its locally destructive behavior and high recurrence rates, malignant transformation of the cystic lining component of OKC is a rare occurrence. In this report, we documented the clinical and radiographic features of a highly destructive maxillary process that demonstrated infiltrative, keratinizing SCC. Interestingly, the complete histopathological examination of the biopsy specimens revealed foci where nests of SCC were noted arising from OKC lining demonstrating varying degrees of dysplasia. The majority of maxillary SCCs arise from sinonasal mucous membranes; the identification of foci with the OKC lining, as described above, demonstrates an example of SCC likely arising from the lining of a pre-existing benign odontogenic lesion.
Malignancies of the sinonasal region represent less than 0.8% of all malignant neoplasms, and approximately 3% of all malignant neoplasms in the upper aerodigestive tract.1213 The majority of them are SCCs, representing >65% of all sinonasal malignancies; the majority of SCCs are of the keratinizing squamous variety.1314 Other destructive processes that arise and involve the sinonasal region and nasopharynx include the following: malignant neoplasms arising from the submucosal salivary glands (adenocarcinomas); nasopharyngeal carcinoma (related to Epstein-Barr virus); olfactory neuroblastoma, sinonasal undifferentiated carcinoma; neuroendocrine malignancies (Ewing sarcoma and primitive neuroectodermal tumor); and malignant lymphoproliferative disease.12131415 In rare instances, maxillary OKCs and other benign destructive odontogenic tumors (such as ameloblastoma and myxoma), have the potential to cause substantial sinonasal bone destruction. OKCs have been known to involve the sinuses, sinonasal skeleton, and on occasion the floor of the orbit; in such cases, they can cause epiphora, proptosis, nasolacrimal duct obstruction, and globe dystopia. The presenting clinical signs and symptoms in patients with the above lesions may include an expansile mid-facial mass, sinonasal obstruction, epistaxis, pain, and/or paresthesia. Patients with destructive odontogenic tumors may present with an unerupted/impacted tooth and intraoral swelling. Each of the above lesions is associated with radiographic features suggestive of either a malignancy or infection/inflammatory disease: lytic radiolucencies with ill-defined, non-corticated borders, destruction or ‘blow-out’ of the sinonasal floor and walls, nasal septal deviation and/or destruction, and involvement of the orbital floor; a varying degree of opacity may be observed depending on the tissue comprising the lesion. Our patient presented with a relatively rapidly progressing maxillary mass with associated pain in the canine/premolar region. The clinical and radiographic findings (plain films/CT) were strongly suggestive of a rapidly progressing, aggressive, malignant neoplasm. Histopathological examination confirmed the presence of SCC, and the discovery of OKC lining within the sections was somewhat serendipitous.
Until recently, OKCs were classified as a developmental odontogenic cyst with locally destructive behavior. Studies into the behavior and pathogenesis of OKCs revealed mutations associated with the PTCH gene, primarily in syndromic OKCs and only in very few sporadic OKCs.161718 These genetic changes that are frequently germline/congenitally acquired are significantly different from the somatic/acquired mutations observed in SCC: allelic imbalance of the 9p21 locus, dysregulation/deletion of p53, and dysregulation of the epidermal growth factor pathway. Although exceedingly uncommon, malignant transformation within the cystic lining of primarily odontogenic processes has been documented in the literature.101920212223 These SCCs have been termed primary intraosseous odontogenic carcinomas.24 The frequency of carcinomatous transformation within odontogenic cysts is as low as 0.01% to 0.02%;25 the extant literature includes 7 reports of SCC arising within the lining of OKC.891920262728 Ward and Cohen29 proposed 3 possible explanations of the presence of SCC within odontogenic cystic lining: 1) a pre-existing cyst secondarily involved with an SCC of unrelated origin; 2) a primary SCC with areas of cystic degeneration; and 3) a primary odontogenic cyst in which the lining underwent malignant carcinomatous changes. Furthermore, Gardner and Waldron24 proposed additional criteria that aid in the diagnosis of SCC arising within the OKC lining. To summarize their criteria, in order to rule out the likelihood of a primary sinonasal, oral mucosal, or metastatic SCC, it is essential to see a focus of SCC arising directly from an area of diagnostic cyst lining pathognomonic for OKC. This was the case in our patient’s initial biopsy specimens; SCC was noted arising within the OKC lining.
The patient reported in this case presented with an infiltrative SCC that caused substantial destruction of the sinonasal and mid-facial skeleton, including the floor of the orbit. The coincidental finding of OKC lining within the tumor played no part in the staging and final treatment planning, with the diagnosis of SCC taking precedence over the primary odontogenic lesion. Additional imaging was obtained to aid in the staging of the tumor, and to rule out loco-regional or distant metastasis and lymph node involvement. Surgical treatment, including wide local resection, en bloc excision or radical resection of the involved bone, is the management of choice in patients with intraosseous SCC.263031 Combined radiation therapy is frequently advocated in extensive tumors that present a surgical challenge, as was the case with our patient. Routine neck dissection is generally not indicated given the low frequency of cervical metastasis noted with maxillary SCCs.32 Given the challenges posed by the intricate osseous structure of the mid-facial skeleton, and the nature of local tumor infiltration, the prognosis in patients with maxillary SCCs is relatively poor, with a 5-year survival rate ranging from 30% to 40%.31 In conclusion, we have presented the clinical, radiographic, and histopathological features of a maxillary sinonasal SCC arising within the lining of a pre-existing OKC. We discussed the clinical and radiographic features of destructive maxillary lesions, as well as differential diagnostic considerations. While acknowledging that the vast majority of OKCs are benign, with the potential to be locally destructive, the current report illustrates the potential for carcinomatous transformation within the lining of OKCs.
Figures and Tables
Fig. 1
Preoperative panoramic radiograph demonstrates extensive bone destruction in the right maxilla. The lesion is ill-defined, with ‘blow-out’ of much of the right antrum, also involving the right orbital floor. The maxillary canine is impacted by a pericoronal radiolucency that appears well defined.
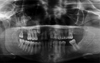
Fig. 2
A and B. Axial bone window and coronal soft tissue algorithms of CT show the impacted right maxillary canine surrounded by a large, well defined, low intensity lesion growing more along the bone than buccal palatal expansion, extending through the palate and expanding and thinning the buccal cortical border adjacent to the right canine. C and D. Axial bone window and coronal soft tissue algorithm of CT show erosion of much of the floor, anterior and lateral walls of the right maxillary sinus, obliteration of the entire right antral cavity and invasion of the surrounding soft tissue by the lesion. The right lateral nasal wall adjacent to inferior concha and ipsilateral orbital floor are also eroded and edema of the superficial soft tissue on the right side is evident.
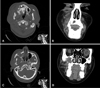
Fig. 3
Squamous cell carcinoma arising in an odontogenic keratocyst. A. Low-power magnification demonstrates the cyst lining and a satellite cyst within the wall. B. A satellite cyst demonstrates classic odontogenic keratocyst lining epithelium. Note the regimented basal cells and the uniform thickness of the epithelium. C. Odontogenic keratocyst lining demonstrates dysplastic changes. Note the nests of SCC arising from and contiguous with the cyst lining. D. A high-power view shows the infiltrative squamous cell carcinoma.
Acknowledgements
The authors would like to thank Dr. E. Natarajan for his contribution to the pathology section of this case report.
References
1. Maurette PE, Jorge J, de Moraes M. Conservative treatment protocol of odontogenic keratocyst: a preliminary study. J Oral Maxillofac Surg. 2006; 64:379–379.

2. Habibi A, Saghravanian N, Habibi M, Mellati E, Habibi M. Keratocystic odontogenic tumor: a 10-year retrospective study of 83 cases in an Iranian population. J Oral Sci. 2007; 49:229–235.

3. Sharif FNj, Oliver R, Sweet C, Sharif MO. Interventions for the treatment of keratocystic odontogenic tumors (KCOT, odontogenic keratocyst (OKC)). Cochrane Database Syst Rev. 2010; 9:CD008464.
4. Mendes RA, Carvalho JF, Van der Waal I. Characterization and management of the keratocystic odontogenic tumor in relation to its histological and biological features. Oral Oncol. 2010; 46:219–225.
5. Soskolne WA, Shear M. Observations on the pathogenesis of primordial cysts. Br Dent J. 1967; 123:321–326.
6. Chirapathomsakul D, Sastravaha P, Jansisyanont P. A review of odontogenic keratocysts and the behavior of recurrences. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006; 101:5–10.

7. Shear M. The aggressive nature of the odontogenic keratocyst: is it a benign cystic neoplasm? Part 1. Clinical and early experimental evidence of aggressive behaviour. Oral Oncol. 2002; 38:219–226.

8. Browne RM, Gough NG. Malignant change in the epithelium of lining odontogenic cysts. Cancer. 1972; 29:1199–1207.
9. Fanibunda K, Soames JV. Malignant and premalignant change in odontogenic cysts. J Oral Maxillofac Surg. 1995; 53:1469–1472.

10. Falaki F, Delavarian Z, Salehinejad J, Saghafi S. Squamous cell carcinoma arising from an odontogenic keratocyst: a case report. Med Oral Patol Oral Cir Bucal. 2009; 14:E171–E174.
11. Lee JW, Gates R, Wignall A. Squamous cell carcinoma arising from a keratocystic odontogenic tumor. Otolaryngol Head Neck Surg. 2011; 145:356–357.

12. Batsakis JG, Rice DH, Solomon AR. The pathology of head and neck tumors: squamous and mucous-gland carcinomas of the nasal cavity, paranasal sinuses, and larynx, part 6. Head Neck Surg. 1980; 2:497–508.

13. Slootweg PJ, Richardon M. Squamous cell carcinoma of the upper aerodigestive system. In : Gnepp DR, editor. Diagnostic surgical pathology of the head and neck. 2nd ed. Philadelphia, PA: Saunders Elsevier;2009. p. 45–110.
14. Syrjänen KJ. HPV infections in benign and malignant sinonasal lesions. J Clin Pathol. 2003; 56:174–181.
15. Mills SE. Neuroectodermal neoplasms of the head and neck with emphasis on neuroendocrine carcinomas. Mod Pathol. 2002; 15:264–278.

16. Agaram NP, Collins BM, Barnes L, Lomago D, Aldeeb D, Swalsky P, et al. Molecular analysis to demonstrate that odontogenic keratocysts are neoplastic. Arch Pathol Lab Med. 2004; 128:313–317.

17. Gomes CC, Diniz MG, Gomez RS. Review of the molecular pathogenesis of the odontogenic keratocyst. Oral Oncol. 2009; 45:1011–1014.

18. Henley J, Summerlin DJ, Tomich C, Zhang S, Cheng L. Molecular evidence supporting the neoplastic nature of odontogenic keratocyst: a laser capture microdissection study of 15 cases. Histopathology. 2005; 47:582–586.

19. Yoshida H, Onizawa K, Yusa H. Squamous cell carcinoma arising in association with an orthokeratinized odontogenic keratocyst. Report of a case. J Oral Maxillofac Surg. 1996; 54:647–651.
20. Minić AJ. Primary intraosseous squamous cell carcinoma arising in a mandibular keratocyst. Int J Oral Maxillofac Surg. 1992; 21:163–165.

21. Areen RG, McClatchey KD, Baker HI. Squamous cell carcinoma developing in an odontogenic keratocyst. Report of a case. Arch Otolaryngol. 1981; 107:568–569.

22. Makowski GJ, McGuff S, Van Sickels JE. Squamous cell carcinoma in a maxillary odontogenic keratocyst. J Oral Maxillofac Surg. 2001; 59:76–80.

23. Chaisuparat R, Coletti D, Kolokythas A, Ord RA, Nikitakis NG. Primary intraosseous odontogenic carcinoma arising in an odontogenic cyst or de novo: a clinicopathologic study of six new cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006; 101:194–200.

24. Waldron CA, Mustoe TA. Primary intraosseous carcinoma of the mandible with probable origin in an odontogenic cyst. Oral Surg Oral Med Oral Pathol. 1989; 67:716–724.

25. Meara JG, Shah S, Li KK, Cunningham MJ. The odontogenic keratocyst: a 20-year clinicopathologic review. Laryngoscope. 1998; 108:280–283.
26. Sato T, Kamiya Y, Tochihara S, Tochihara S, Toyoda N, Asada K, et al. Primary intraosseous squamous cell carcinoma of the mandible: report of a case and review of the recent literature. Oral Med Pathol. 2006; 11:83–88.
27. McDonald AR, Pogrel MA, Carson J, Regezi J. p53-positive squamous cell carcinoma originating from an odontogenic cyst. J Oral Maxillofac Surg. 1996; 54:216–218.

28. Anand VK, Arrowood JP Jr, Krolls SO. Malignant potential of the odontogenic keratocyst. Otolaryngol Head Neck Surg. 1994; 111:124–129.

30. Bodner L, Manor E, Shear M, van der Waal I. Primary intraosseous squamous cell carcinoma arising in an odontogenic cyst: a clinicopathologic analysis of 116 reported cases. J Oral Pathol Med. 2011; 40:733–738.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download