Abstract
Dentinogenesis imperfecta is a dominant autosomal hereditary disorder of dentin formation that affects the deciduous and permanent teeth. Its etiology is characterized by inadequate cell differentiation during odontogenesis. The clinical characteristics of dentinogenesis imperfecta are discolored teeth with a translucency that varies from gray to brown or amber. Radiographically, the teeth exhibit pulp obliteration, thin and short roots, bell-shaped crowns, and periapical bone rarefaction. The aim of this report was to present a case of dentinogenesis imperfecta type II that was followed up over a 17-year period. This report also presents scanning electron microscopy images of the enamel and dentin, showing that both were altered in the affected teeth. The disease characteristics and the treatments that were administered are reported in this study to guide dentists with respect to the need for early diagnosis and adequate follow-up to avoid major sequelae.
Dentinogenesis imperfecta is a rare tooth development disorder with a dominant autosomal hereditary character, with no direct link to gender, and in most cases, it occurs as a single abnormality.1 Dentinogenesis imperfecta affects both the deciduous and permanent teeth, and can appear in association with osteogenesis imperfecta.
Dentinogenesis imperfecta is divided into 3 subgroups: types I, II, and III.1 Type I is a defect associated with osteogenesis imperfecta; type II, also called hereditary opalescent dentin, is a more common genetic tooth disorder; and type III is the Brandywine isolate type found most commonly in Brandywine, MD, USA.123 Dentinogenesis imperfecta type II is a dominant hereditary tooth disorder, with an estimated incidence of 1 : 8000.45
The teeth affected by dentinogenesis imperfecta contain dentin with an irregular structural formation that is less mineralized than normal. In a clinical examination, these teeth exhibit an amber coloration, varying from yellow to brown and from gray to blue, with an opalescent, translucent shine. Radiographically, in both the deciduous and permanent teeth, dentinogenesis imperfecta presents root canals and pulp chambers with progressive obliteration due to continuous and disordered dentin deposition by ondontoblasts.26 The tooth crowns are short and have a bulb or signet form, while the roots, in addition to being short, are constricted.6
The enamel tends to chip away from the dentin, exposing dentin dysplasia, which can lead to rapid attrition until the alveolar ridge is reached.78 In such cases, the treatment for this condition involves the structural protection of the affected teeth to avoid the development of caries and wear due to abrasion, as well as to promote the maintenance of the vertical dimension of occlusion. Further, fixed total crown prostheses, total prostheses, or overdenture may be necessary.
The psychosocial impact of this condition and the limitations in existing therapeutic strategies can hamper treatment. Therefore, the aim of this case report was to describe the clinical, radiographic, and ultrasonographic characteristics of the teeth of a patient with dentinogenesis imperfecta type II, who underwent 17 years of clinical follow-up.
A 3-year-old girl was admitted to the Department of Pediatric Dentistry, Pontific Catholic University of Minas Gerais, with brown discoloration and advanced wear in the primary teeth. She was in good health, and her medical history was non-contributory. The intraoral exam showed pulp exposure and the presence of periapical fistulae. The patient's radiographs exhibited teeth with shortened and worn crowns, thin roots with constriction in the cervical region, and total or partial obliteration of the pulp chamber and the root canals of the previous deciduous teeth. The clinical and radiographic images of the patient, in association with her family's medical history, supported the diagnosis of dentinogenesis imperfecta type II, as described by Shields et al.,1 with a pedigree covering 3 generations (Fig. 1). The treatment plan set up for this patient had the aim of preserving the tooth structures because of their susceptibility to accentuated wear of the tooth crowns.
At 7 years of age, the patient returned to the clinic. An intraoral exam showed the beginning of the eruption of the permanent first molars, an early loss of deciduous teeth, an intraoral abscess, and deciduous teeth with exposed dentin. To protect the permanent first molars from early wear, a photopolymerizable composite resin (Command Universal, Kerr Corporation, Orange, CA, USA) was added to the occlusal surface, which also allowed an increase in the vertical dimension of the lost occlusion due to the wear of the deciduous molars. Some months later, temporary stainless steel crowns were cemented to the 4 permanent first molars to protect the integrity of the natural crowns (Fig. 2). Aimed at obtaining occlusal balance without overloading these molars, photopolymerizable composite resin (Command Universal and Herculite XR, Kerr Corporation, Orange, CA, USA) was added to the mandibular deciduous molars. Under the supervision of an orthodontist, the decision was made to extract a number of deciduous teeth. Upon treatment completion, a new stage of follow-up began until the complete eruption of the maxillary and mandibular incisors.
At 9 years of age, a complete crown was manufactured using another type of photopolymerizable composite resin (Multifill and Durafill, Heraeus Kulzer, South Bend, IN, USA), by a direct method, for the maxillary central and lateral incisors and the mandibular central incisors. At the beginning of the eruption of the permanent pre-molars, an acrylic retainer with a bite plane was inserted to survey the bite and free up space for the pre-molars to erupt.
After a year, the use of the Hawley retainer was suspended, and the maxillary and mandibular pre-molars were again covered by a complete crown manufactured using photopolymerizable composite resin. At the same time, the temporary stainless steel crowns were removed from the permanent first molars to create a more physiological space for them. A new stage of observation began.
At 12 years of age, the patient returned for a follow-up visit. Crowns for the first and second molars were manufactured using photopolymerizable composite resin in the same manner as those manufactured for the incisors and pre-molars. Later, the permanent canines were also covered with resin. Another observation period started.
At 18 years of age, it was necessary to extract the patient's maxillary third molars and the right mandibular third molar, as these were unerupted and impacted. The left mandibular third molar was not extracted, as it was well-positioned in the dental arch. After 17 years of follow-up, at 20 years of age, the patient underwent her final evaluation. All the permanent teeth were covered with photopolymerizable resin and were appropriately positioned (Fig. 3). No periodontal alterations were found, and the esthetics were adequate. The third molars had already been extracted.
The extracted third molars were sent for analysis by scanning electron microscopy (SEM), using Stereoscan 440 (Leica Cambridge, Cambridge, UK) with a magnification of up to ×1000, and were compared with normal teeth. The SEM images showed changes in both the enamel and the dentin of the teeth affected by dentinogenesis imperfecta. While the dentin and the enamel of a normal tooth appear as a compact mass of hydroxyapatite crystals forming prisms of the enamel in an organized and guided manner (Fig. 4A), the enamel structure of the teeth affected by dentinogenesis imperfecta showed changes throughout the enamel, with the absence of parallelism in the prismatic enamel crystals and variation in crystalline size (Fig. 4B). With respect to the microstructure of the dentin, we observed, in the SEM images, differences in the size, diameter, quantity, and direction of the dentin tubules (Fig. 4C). The SEM images of the dentin structure of the affected teeth showed obliteration and a reduction of the quantity of the dentin tubules, with the presence of many atubular areas (Fig. 4D).
This clinical case describes a 17-year follow-up of a patient with dentinogenesis imperfecta type II, which was successfully treated when the patient was 3 to 19 years of age. SEM images of the affected enamel and dentin were also analyzed.
Kim and Simmer4 described the etiology of dentinogenesis imperfecta as a defect in the gene that codes for most dentin proteins, including collagen type I. The enamel appears to be normal; however, it tends to detach and fracture because of occlusal stress, thus exposing the dentin, which exhibits a soft consistency and, therefore, wears quickly and can lead to the alveolar process.59 The presence of a fracture in the enamel is attributed to the abnormality of the cementoenamel junction, which is smooth rather than scalloped, and to deficient calcification of the dentin, which mainly affects the occlusal surface of the posterior teeth and the incisal edges of the anterior teeth.10 Witkop5 also described the presence of hypomature enamel in one-third of the teeth. The most appropriate treatment was the preservation of the tooth structure to ensure the normal growth of the facial bones and the temporomandibular joints.9
In the present clinical case, all clinical characteristics consistent with those of dentinogenesis imperfecta type II were observed, such as light brown deciduous teeth and intense wear of the crowns at the alveolar level. The radiographs showed teeth with shortened and worn crowns, thin roots with constriction in the cervical region, and total or partial obliteration of both the pulp chamber and the root canals.
Some authors have reported that attrition reduces the incidence of carious lesions; however, periapical lesions are very common because of the quick exposure of the pulp to attrition.6111213 The patient described in this clinical case presented many carious lesions until 7 years of age because of poor oral hygiene and an inappropriate diet. After intense work with the family on oral prevention and providing thorough instructions about diet and oral hygiene, plaque control, and fluoride use, the patient maintained good oral health up to adulthood.
Knowledge of the clinical and radiographic findings that are in accordance with this medical condition are essential to planning early treatment in such a way that the tooth structure is preserved to the maximum possible extent. This also prevents possible sequelae, in addition to tooth loss, with the goal of diminishing patient discomfort and providing a better quality of life. In this clinical case, the main measure taken was to preserve the tooth structure, preventing the permanent molars from entering into occlusion, through temporary stainless steel crowns and a later addition of photopolymerizable resins on the clinical crowns of all posterior teeth to maintain the vertical dimensions.
Changes in the dentin observed in the SEM images of patients with dentinogenesis imperfecta have been reported by many authors.61213141516 The presence of an atubular area in the dentin with reduced mineralization and a reduced number of odontoblasts are characteristic findings in the literature. However, changes in the enamel have rarely been described in the literature. Gallusi et al.16 observed few structural changes in the enamel and concluded that there was no relationship between enamel morphology and dentinogenesis imperfecta type II. Leal et al.13 reported cracks in the enamel of the affected primary teeth. Wieczorek and Loster12 showed granular remnants of the enamel when studying the ultrastructure of the permanent teeth affected by dentinogenesis imperfecta. Devaraju et al.6 reported that changes in dentin formation resulted in changes in the enamel, such as hypoplastic and hypocalcified areas within the enamel rods. In contrast, the microscopic study of Davis et al.14 of primary teeth with dentinogenesis imperfecta revealed enamel with normal morphology and calcification. Our patient's permanent tooth enamel ultrastructure, analyzed through SEM images, showed an absence of parallelism in the prismatic enamel crystals and variations in the crystal size. The disturbances of the enamel and dentin revealed by the SEM images illustrate structural defects that explain the low hardness and the low attrition resistance of the enamel and, consequently, the difficulty of restoration and restoration loss observed in patients with dentinogenesis imperfecta.612
Dentinogenesis imperfecta is a disorder that causes serious damage to the patient, not only esthetically but also psychologically. Pulp obliteration, changes in color, accentuated wear, and loss of enamel are only some of the forms of damage resulting from this medical condition. The hereditary character of the disease can aid dental professionals in their clinical conduct, helping them guide relationships with the family members in such a way as to minimize the possible trauma suffered by the patient. The early detection of the disease is essential for choosing appropriate appropriate treatment with the goal of preserving as much of the tooth structure of the affected teeth as possible. In this sense, early diagnosis favors a good prognosis and minimizes sequelae, improving the esthetics and the quality of life of patients with dentinogenesis imperfecta.
Figures and Tables
Fig. 1
Pedigree of dentinogenesis imperfecta in the patient's family medical record, covering 3 generations.
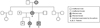
Fig. 2
Radiographic images of the 7-year-old patient. Temporary stainless steel crowns are present in the permanent first molars, and direct composite resin restorations are present in the primary mandibular molars.
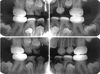
Fig. 3
Panoramic radiograph of the patient at 20 years of age, showing obliterated pulp chambers, shortened crowns restored with resin, and thin roots with constriction in the cervical region.
Fig. 4
Scanning electron microscopy images (×1000) show the enamel and dentin of both a normal tooth and a tooth affected by dentinogenesis imperfecta. A. Parallelism of the prismatic enamel crystals can be observed. B. Absence of parallelism in the prismatic enamel crystals is found. C. Normal dentin structure with a tubular direction throughout its entire structure is found. D. Affected dentin structure with the obliteration and reduction of the quantity of dentin tubules is seen.

References
1. Shields ED, Bixler D, El-Kafrawy AM. A proposed classification for heritable human dentine defects with a description of a new entity. Arch Oral Biol. 1973; 18:543–553.

2. MacDougall M, Dong J, Acevedo AC. Molecular basis of human dentin diseases. Am J Med Genet A. 2006; 140:2536–2546.

3. Witkop CJ Jr. Amelogenesis imperfecta, dentinogenesis imperfecta and dentin displasia revisted: problems in classification. J Oral Pathol. 1988; 17:547–553.
5. Witkop CJ Jr. Hereditary defects of dentin. Dent Clin North Am. 1975; 19:25–45.
6. Devaraju D, Devi BY, Vasudevan V, Manjunath V. Dentinogenesis imperfecta type I: a case report with literature review on nomenclature system. J Oral Maxillofac Pathol. 2014; 18:Suppl 1. S131–S134.

7. Knezević A, Tarle Z, Pandurić V. Esthetic reconstruction of teeth in patient with dentinogenesis imperfecta - a case report. Coll Antropol. 2006; 30:231–234.
8. Modesto A, Alves AC, Vieira AR, Portella W. Dentinogenesis imperfecta type II: case report. Braz Dent J. 1996; 7:47–52.
9. Ranta H, Lukinmaa PL, Waltimo J. Heritable dentin defects: nosology, pathology, and treatment. Am J Med Genet. 1993; 45:193–200.

10. Singh M, Singh S. Hereditary opalescent dentin - a case report. J Indian Soc Pedod Prev Dent. 2004; 22:144–147.
11. Koenig MM, Taylor DT. Hereditary opalescent dentin. ASDC J Dent Child. 1973; 40:461–466.
12. Wieczorek A, Loster J. Dentinogenesis imperfecta type II: ultrastructure of teeth in sagittal sections. Folia Histochem Cytobiol. 2013; 51:244–247.

13. Leal CT, Martins LD, Verli FD, de Souza MA, Ramos-Jorge ML. Case report: clinical, histological and ultrastructural characterization of type II dentinogenesis imperfecta. Eur Arch Paediatr Dent. 2010; 11:306–309.
14. Davis GR, Fearne JM, Sabel N, Noren JG. Microscopic study of dental hard tissues in primary teeth with dentinogenesis imperfecta type II: correlation of 3D imaging using X-ray microtomography and polarising microscopy. Arch Oral Biol. 2015; 60:1013–1020.

15. Regezi JA, Sciubba JJ, Jordan RC. Oral pathology: clinical pathologic correlations. 7th ed. Philadelphia: Saunders;2017. p. 373–388.
16. Gallusi G, Libonati A, Campanella V. SEM-morphology in dentinogenesis imperfecta type II: microscopic anatomy and efficacy of a dentine bonding system. Eur J Paediatr Dent. 2006; 7:9–17.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download