Abstract
Purpose
To evaluate the upper airway dimensions of obstructive sleep apnea (OSA) and control subjects using a cone-beam computed tomography (CBCT) unit commonly applied in clinical practice in order to assess airway dimensions in the same fashion as that routinely employed in a clinical setting.
Materials and Methods
This was a retrospective analysis utilizing existing CBCT scans to evaluate the dimensions of the upper airway in OSA and control subjects. The CBCT data of sixteen OSA and sixteen control subjects were compared. The average area, average volume, total volume, and total length of the upper airway were computed. Width and anterior-posterior (AP) measurements were obtained on the smallest axial slice.
Results
OSA subjects had a significantly smaller average airway area, average airway volume, total airway volume, and mean airway width. OSA subjects had a significantly larger airway length measurement. The mean A-P distance was not significantly different between groups.
Conclusion
OSA subjects have a smaller upper airway compared to controls with the exception of airway length. The lack of a significant difference in the mean A-P distance may indicate that patient position during imaging (upright vs. supine) can affect this measurement. Comparison of this study with a future prospective study design will allow for validation of these results.
The National Commission on Sleep Disorders estimates that minimal sleep-disordered breathing affects 7 to 18 million people in the United States, while 2 to 4 million Americans have moderate to severe disease. Obstructive sleep apnea (OSA) is characterized by repetitive episodes of pharyngeal collapse, specifically of the upper airway, in association with an increase in resistance to airflow during sleep.123 OSA is diagnosed when there are sleep-related clinical symptoms in the presence of at least five obstructive respiratory events per hour of sleep.45 Alternatively, OSA is diagnosed in the absence of sleep-related clinical symptoms when there are ≥15 obstructive respiratory events per hour of sleep.45 Obstructive respiratory events during sleep are reported according to the apneahypopnea index (AHI) or respiratory disturbance index (RDI).4 Mild OSA is defined as a RDI≥5 and <15 while moderate and severe disease are defined as a RDI≥15.4 OSA is classified as severe when the RDI is >30.4 OSA is the result of a complex interaction between anatomic factors (round airway, soft palate length and volume, upper airway length, pharyngeal fat deposits, adenotonsillar hypertrophy, tongue volume, class II skeletal profile, and morphological deviations of the cervical spine), sleep-related factors, and central nervous system control over ventilation.26789 Structural and non-structural risk factors for OSA are summarized in Table 1.
Despite the high incidence of OSA, it is estimated that up to 93% of women and 82% of men may have undiagnosed moderate to severe disease.5 Therefore, it is not unlikely that a patient with undiagnosed OSA may present for a routine visit in a dental office. Unfortunately, OSA is associated with multiple comorbidities including hypertension, obesity, gastroesophageal reflux, impotence, depression, and higher rates of cardiovascular and cerebrovascular morbidity and mortality.121011 Therefore, recognition of and screening for OSA during a routine dental visit is of vital importance.
Cone-beam computed tomography (CBCT) is a commonly used three-dimensional imaging technique introduced to dentistry in 1998.12 The Next Generation iCAT CBCT unit (Imaging Sciences International, Hatfield, PA, USA), which was used in this study, is capable of acquiring quality images at a radiation dose equivalent to approximately one-half of the dose associated with conventional two-dimensional imaging (i.e. a full mouth series of intraoral radiographs).13 When utilizing a large field of view (FOV) protocol the upper airway is visible within the CBCT volume and thus CBCT is a useful diagnostic tool for evaluation of the airway. Assessment of the upper airway in individuals with OSA is essential, as they have reportedly smaller upper airways than individuals without OSA.814 For example, using CBCT, Enciso and colleagues found that the presence and severity of OSA is associated with a narrow lateral dimension of the airway.15 Similarly, using medical CT, Mayer and colleagues reported a decrease in the transverse width of the oropharynx in OSA subjects.16 Furthermore, evaluation of the upper airway is essential due to the reported increase in the frequency of airway collapse in individuals with narrower and longer airways.2
Due to the high incidence of OSA and widespread use of CBCT, it is possible that dental patients at risk for OSA (due to narrower and longer airways) may be identified on CBCT examinations originally acquired for other diagnostic purposes. Most of the commercial dental CBCT units currently in use acquire imaging with the patient in an upright position. For this reason, there is a need to evaluate the morphology of the airway of OSA subjects in the same manner. Unfortunately, numerous articles on the dimensions of OSA subjects have evaluated these patients in a supine position, which is not commonly applied in clinical dentistry. It has also been suggested that CBCT imaging of patients in the supine position is not appropriate because it does not simulate conditions during sleep.17
The purpose of this retrospective study was to evaluate the upper airway dimensions of OSA and control subjects using a CBCT unit, commonly utilized in clinical practice, which employs an upright patient position during image acquisition.
This was a retrospective study of CBCT imaging. All CBCT examinations were performed with the iCAT Next Generation unit (Imaging Sciences International, Hatfield, PA, USA). During image acquisition with this unit, the patient is positioned upright and seated throughout exposure. Inclusion criteria for the study subjects consisted of individuals with OSA and individuals without OSA (control group). The gender and age of the control group and OSA group subjects were matched as closely as possible. The mean age was 43.3 years in the OSA group (range 21-68) and 44.6 years in the control group (range 28-72). The presence of OSA was confirmed with a sleep study (polysomnography (PSG) in 11 cases; home sleep test (HST) in 5 cases). All OSA subjects were diagnosed with moderate to severe OSA. In order for the CBCT scan to be included in the study, the size of the field of view needed to cover the nasopharynx and oropharynx and appropriate tongue position had to be attained. Tongue positioning plays an important role in the size of the airway, as evidenced by a decrease in airway size due to a retrodisplacement of the tongue base observed by Camacho and colleagues in supine patients.10 Therefore, CBCT studies displaying a posterior positioning of the tongue, as evidenced by air space between the tongue and hard palate, were not included in the study. Additional exclusion criteria consisted of edentulous patients, in whom mandibular jaw position and subsequent tongue position may be affected when the prosthesis is removed from the mouth during image acquisition, malocclusions (for example significant mandibular retrognathia or prognathia), and control subjects not matched to the age and gender of the OSA group. Application of the inclusion and exclusion criteria resulted in a total sample size of 32 (16 subjects+ 16 controls). The study was approved by our University Institutional Review Board (study identification number Pro00000894).
The CBCT images were exported as DICOM (.dcm) files and then imported into the software program Analyze 10.0 (AnalyzeDirect, Overland Park, KS, USA) for analysis of the airway. A coding system was used to anonymize the data by assigning a random two-digit number to each study. The upper airway affected by OSA is frequently defined as the soft tissue region bounded by the nasopharynx superiorly and the epiglottis inferiorly.2 Therefore, the oropharynx from hard palate to epiglottis was isolated from the data for analysis by manual segmentation of each axial slice from the surrounding soft tissue using thresholding, or setting the upper and lower grey level values of the area of interest (upper airway). The same individual performed all segmentation. Figure 1 demonstrates the segmented airway with three-dimensional reconstruction. Once the airway was isolated, the software computed the area (mm2) and volume (mm3) of each axial slice for the entire isolated portion of the airway. These area and volume measurements were used to identify the smallest axial slice, and width and anterior-posterior (A-P) dimension measurements were then carried out on the smallest axial slice in each CBCT study (Fig. 2). The average of multiple measurements was recorded as the value representing the width and AP dimension. All linear measurements were acquired by the same individual. The total number of axial slices segmented from the hard palate to the epiglottis were used to calculate the airway length.
The Shapiro-Wilk test was used to confirm the assumption of normality in each group (OSA and control). If the normality assumption was valid in both groups, the unequal variance t-test was used to compare the means of the two groups. If the normality assumption was violated in either group, the Wilcoxon rank-sum test was used to compare the groups. All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA). All statistical tests were two-tailed and were performed using a significance level of 0.05. Data were summarized using mean±standard deviation (S.D.).
The Shapiro-Wilk test indicated that the assumption of normality was reasonable for A-P distance, width (lateral measurement), total airway volume, and airway length in both groups (p>0.10 for all tests). The normality assumption was violated in the OSA group for average airway area (p=0.0032) and average airway volume (p=0.0032).
The data for the width, A-P distance, area, volume, and airway length in each group are summarized in Tables 2, 3, 4. The unequal variance t-test indicated that the mean width was significantly smaller in the OSA group than in the control group (p=0.0011); however, the difference between groups in terms of mean A-P distance was not significant (p=0.3049). The Wilcoxon rank-sum test indicated that both average airway area and average airway volume were significantly smaller in the OSA group than in the control group (p=0.0014 for both comparisons). The unequal variance t-test indicated that the mean total airway volume was significantly smaller in the OSA group than in the control group (p=0.0053), and that the mean airway length was significantly larger in the OSA group than in the control group (p=0.0139). Figure 3 demonstrates the difference in upper airway width between an OSA subject and a control subject.
The A-P distance and the width of the minimum surface area of the oropharynx are commonly used to evaluate the upper airway.3 A recent systematic review revealed that the most common measurements of the airway used to evaluate OSA subjects with CBCT included total volume and minimum cross-sectional area, followed by area and lateral and anterior-posterior linear measurements.17 Accordingly, in our study we evaluated the width (lateral) and A-P distance of the smallest axial cross sectional slice, the total airway volume, and the average area and volume of the airway. Additionally, we evaluated the length of the upper airway because OSA subjects have reportedly longer airways and there is an increase in the frequency of airway collapse in those with narrower and longer airways.2
Enciso and colleagues determined using CBCT that the likelihood of OSA is 3.9 times higher when the lateral dimension of the oropharynx measures less than 17 mm.15 The mean measurement of airway width in our subjects was 15.79 mm. Hora et al., 2007 assessed the airway in OSA patients and controls using MRI, and found the airway width to be smaller in OSA patients and in fact determined that the width of the airway at the retroglossal level was an independent predictor for OSA.18 In our study the width of the smallest cross sectional slice of the oropharynx was significantly smaller in OSA subjects than controls, which is consistent with these published studies.
Conversely, our findings differed from those of previous studies when the anterior-posterior distance of the airway was considered. Ogawa and colleagues found that the A-P dimension was smaller in OSA subjects compared with controls,3 and Schwab and colleagues found that the A-P dimension was significantly smaller in OSA subjects than controls when measuring the retropalatal region of the airway.8 The differences in airway measurements in the latter study were maintained after controlling for sex, ethnicity, age, craniofacial size, and parapharyngeal fat.8 In contrast, there was no statistical difference in A-P measurement between the OSA and control group in our study. One main difference between these studies and ours is that our subjects were imaged in the upright position while their subjects were imaged in the supine position. A recent study conducted by Camacho and colleagues was the first to compare airway morphology between upright and supine patient positions using CBCT, and found that the minimum cross-sectional area decreased from 124±29 mm2 to 30±5 mm2 when the patient was scanned in the supine position.10 The authors attributed this decrease to a retro-displacement of the base of the tongue, palate, and epiglottis when the patients were positioned supinely during CBCT acquisition.10 Additionally, they observed that the tip of the tongue was posteriorly positioned when patients were positioned supinely.10 Others have reported similar findings, relating a decrease in anterior-posterior airway dimension to supine positioning and the resultant narrowing of the velopharynx, relaxation of the soft palate and tongue, or change in hyoid bone position.1719 Therefore, it is possible that the A-P distance of the airway may register at a smaller value when patients are imaged in the supine position.
Finally, we assessed the total mean airway volume, the average area and volume, and the airway length. Each of these measures was significantly smaller in the OSA group compared to the control group, with the exception of airway length, which was significantly larger. Schwab and colleagues found that in the retropalatal region, the airway volume and average airway area per slice remained significantly smaller in OSA subjects after controlling for sex, ethnicity, age, craniofacial size, and parapharyngeal fat.8 Therefore, the smaller the retropalatal airway volume and area, the higher the risk of developing OSA.8 In addition to smaller airway measurements, OSA subjects have been found to have a significantly longer airway relative to controls.14 Smaller airways in OSA subjects can also be demonstrated in the treatment outcomes for OSA subjects. For example, Abramson and colleagues found that a cohort of OSA subjects that underwent maxillomandibular advancement and genial tubercle advancement showed an increase in airway volume post-surgery.14 The majority of the patients in this study reported no symptoms after surgery and their postoperative respiratory disturbance index (RDI) at 6 months was statistically significantly lower than preoperative values.14 Interestingly, the only airway value assessed that decreased post-surgery was airway length, although this still remained significantly greater than in control subjects.14 Therefore, the findings in our study are consistent with the published literature.
Strengths of our study include confirmation of OSA with a sleep study (PSG in 11 cases; HST in 5 cases), the close approximation of the OSA and control subject groups for age and gender, and the criteria used for tongue positioning during CBCT scanning. The exclusion of subjects with a posterior tongue position ensured that the A-P distance measurement was not affected by the narrowing of the airway that can occur when the tongue, and consequentially the soft palate, are positioned posteriorly. However, due to the retrospective design of our study and the stringent inclusion and exclusion criteria, our sample size was limited to 32, which could be considered a potential weakness of the study. Nevertheless, the sample size in our study is consistent with numerous other radiographic studies evaluating OSA. Korayem and colleagues used a sample size of 10 cases and 10 controls, with significance set at 5% and power at 70%, to evaluate OSA.6 In a comparison of the upper airway dimensions between upright and supine positions in OSA subjects, Tsuiki et al. in 2003 used a sample size of 15.19 Ogawa and colleagues used CBCT for radiographic analysis of a sample size of 10 OSA subjects and 10 controls, finding that the upper airway in OSA subjects presented with a smaller minimum cross-section area when compared to control subjects.3 Likewise, in two separate studies analyzing airway dimensions in surgically treated OSA subjects, sample sizes of 11 and 20 were used.1420 Thus, the sample size used in our study is in agreement with the published literature.
The retrospective design of the study could also be viewed as a potential weakness, due to the limitations on certain information imposed by this type of study. For example, body mass index (BMI) was not available for control subjects and therefore this statistic was not available for inclusion in the analysis. Additionally, the control group was selected based upon the absence of a positive history of OSA and no report of signs or symptoms of OSA by the subject. Verification of the absence of OSA requires a sleep evaluation, which was not available due to the retrospective design of the study. A prospective study design would allow better control of the weaknesses of the current study. Therefore, this retrospective study will be followed by a prospective study design to analyze airway dimensions in OSA subjects and controls, with access to BMI and additional statistics such as blood pressure, which will allow better control over sample size and patient positioning during radiographic examination.
In conclusion, our study has demonstrated that OSA subjects have a narrower airway width, a smaller total mean airway volume, a smaller average area and volume, and a larger airway length than control subjects. Moreover, the results of our study suggest that the orientation of the patient during image acquisition (upright vs. supine) may affect the A-P distance measurement of the upper airway. A prospective study design is needed to confirm that the width, total mean airway volume, average area and volume, and airway length may be used to identify potential OSA patients when utilizing an upright positioning CBCT unit.
Figures and Tables
Fig. 2
Linear measurements acquired on an obstructive sleep apnea subject. A. Sagittal view denoting the location of the smallest cross-sectional slice. B. Anterior-posterior distance, measured on the axial image. C. Width (lateral), measured on the axial image.
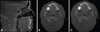
Fig. 3
Comparison of airway width (lateral) between an obstructive sleep apnea (OSA) and a control subject. A. Axial image of an OSA subject depicting the segmented slice. B. Three-dimensional reconstruction of the airway of the OSA subject pictured in A, pictured from the posterior (coronal) view. C. Axial image of a control subject depicting the segmented slice. D. Three-dimensional reconstruction of the airway of a control subject pictured in C. The 3-dimensional reconstruction is pictured from the posterior view of the airway (coronal view).
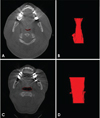
Table 2
Gender, age and linear measurements of width and anterior-posterior (A-P) distance in subjects with obstructive sleep apnea
References
1. Holty JE, Guilleminault C. Maxillomandibular advancement for the treatment of obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev. 2010; 14:287–297.

2. Susarla SM, Thomas RJ, Abramson ZR, Kaban LB. Biomechanics of the upper airway: changing concepts in the pathogenesis of obstructive sleep apnea. Int J Oral Maxillofac Surg. 2010; 39:1149–1159.

3. Ogawa T, Enciso R, Shintaku WH, Clark GT. Evaluation of cross-section airway configuration of obstructive sleep apnea. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007; 103:102–108.

4. Epstein LJ, Kristo D, Strollo PJ Jr, Friedman N, Malhotra A, Patil SP, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009; 5:263–276.
5. Park JG, Ramar K, Olson EJ. Updates on definition, consequences, and management of obstructive sleep apnea. Mayo Clin Proc. 2011; 86:549–555.

6. Korayem MM, Witmans M, MacLean J, Heo G, El-Hakim H, Flores-Mir C, et al. Craniofacial morphology in pediatric patients with persistent obstructive sleep apnea with or without positive airway pressure therapy: a cross-sectional cephalometric comparison with controls. Am J Orthod Dentofacial Orthop. 2013; 144:78–85.
7. Sonnesen L, Jensen KE, Petersson AR, Petri N, Berg S, Svanholt P. Cervical vertebral column morphology in patients with obstructive sleep apnoea assessed using lateral cephalograms and cone beam CT. A comparative study. Dentomaxillofac Radiol. 2013; 42:20130060.

8. Schwab RJ, Pasirstein M, Pierson R, Mackley A, Hachadoorian R, Arens R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003; 168:522–530.

9. Enciso R, Shigeta Y, Nguyen M, Clark GT. Comparison of cone-beam computed tomography incidental findings between patients with moderate/severe obstructive sleep apnea and mild obstructive sleep apnea/healthy patients. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012; 114:373–381.

10. Camacho M, Capasso R, Schendel S. Airway changes in obstructive sleep apnoea patients associated with a supine versus an upright position examined using cone beam computed tomography. J Laryngol Otol. 2014; 128:824–830.

11. Conley RS. Evidence for dental and dental specialty treatment of obstructive sleep apnoea. Part 1: the adult OSA patient and Part 2: the paediatric and adolescent patient. J Oral Rehabil. 2011; 38:136–156.

12. Mozzo P, Procacci C, Tacconi A, Martini PT, Andreis IA. A new volumetric CT machine for dental imaging based on the cone-beam technique: preliminary results. Eur Radiol. 1998; 8:1558–1564.

13. Ludlow JB, Ivanovic M. Comparative dosimetry of dental CBCT devices and 64-slice CT for oral and maxillofacial radiology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008; 106:106–114.

14. Abramson Z, Susarla SM, Lawler M, Bouchard C, Troulis M, Kaban LB. Three-dimensional computed tomographic airway analysis of patients with obstructive sleep apnea treated by maxillomandibular advancement. J Oral Maxillofac Surg. 2011; 69:677–686.

15. Enciso R, Nguyen M, Shigeta Y, Ogawa T, Clark GT. Comparison of cone-beam CT parameters and sleep questionnaires in sleep apnea patients and control subjects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010; 109:285–293.

16. Mayer P, Pepin JL, Bettega G, Veale D, Ferretti G, Deschaux C, et al. Relationship between body mass index, age and upper airway measurements in snorers and sleep apnoea patients. Eur Respir J. 1996; 9:1801–1809.

17. Alsufyani NA, Al-Saleh MA, Major PW. CBCT assessment of upper airway changes and treatment outcomes of obstructive sleep apnoea: a systematic review. Sleep Breath. 2013; 17:911–923.

18. Hora F, Napolis LM, Daltro C, Kodaira SK, Tufik S, Togeiro SM, et al. Clinical, anthropometric and upper airway anatomic characteristics of obese patients with obstructive sleep apnea syndrome. Respiration. 2007; 74:517–524.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download