Abstract
In this report, we describe the incidental finding of an oropharyngeal mass in a patient who presented with a chief complaint of temporomandibular pain. The patient was initially evaluated by an otorhinolaryngologist for complaints of headaches, earache, and sinus congestion. Due to worsening headaches and trismus, he was further referred for the management of temporomandibular disorder. The clinical evaluation was uneventful except for limited mouth opening (trismus). An advanced radiological evaluation using magnetic resonance imaging revealed a mass in the nasopharyngeal/oropharyngeal region. The mass occupied the masticatory space and extended superioinferiorly from the skull base to the mandible. A diagnostic biopsy of the lesion revealed a long-standing human papilloma virus (HPV-16)-positive squamous cell carcinoma of the oropharynx. This case illustrates the need for the timely radiological evaluation of seemingly innocuous orofacial pain.
Orofacial pain is a common complaint in the medical and dental setting.1 This includes complaints of headaches, jaw pain, ear fullness, and clicking sounds in and around the temporomandibular joint (TMJ). A majority of these unclear pain symptoms are commonly associated with a diagnosis of temporomandibular disorder (TMD).1 More concerning is that treatment options may be provided without conclusively identifying the primary underlying cause. Typically, recalcitrant orofacial pain cases are eventually referred to an oral medicine specialist for further evaluation, diagnosis, and management. Eventually it may take several months from the time of the initial complaint for the definitive diagnosis to be established.
Pain in the orofacial region can be potentially caused by infections, trauma, TMJ arthralgia, TMJ articular disc disorder, myofascial pain, psychiatric disorders, atypical facial pain, otalgic pain, and cancer, including hematologic and solid tumors.2 The ease of attributing orofacial pain to the TMJ may be why tumors can initially be considered unlikely in the differential diagnosis until the pain becomes intractable.3 Additionally, the initial use without success of conservative therapeutic management approaches such as physical therapy, analgesics, and muscle relaxants then alerts the clinician to consider the possibility of tumor-associated pain. This is even more the case in the absence of swelling, ulceration, or peripheral lymphadenopathy.
Several case reports have shown that head and neck cancers and metastatic cancer to the head and neck region can present with pain that closely mimics TMD.14 Some of these findings were later diagnosed as nasopharyngeal carcinoma, oral cancer, adenocystic carcinoma, or oropharyngeal carcinoma.245 Due to the anatomical location of the oropharynx and adjacent structures, it is not an area easily visualized by the patient. Furthermore, tumors in this area are painless at an early stage. Therefore, it is not unusual for an oropharyngeal tumor to eventually be detected either incidentally during diagnostic imaging or when surrounding structures are invaded, causing dysphagia, dysphonia, otalgia, or facial pain.
Magnetic resonance imaging (MRI) is an essential tool in diagnosing soft-tissue masses. MRI is more accurate than computed tomography (CT) in delineating between tumor masses and other soft tissues such as muscle, probably because it has fewer artifacts.6
This report presents the case of an incidentally found oropharyngeal mass diagnosed with the aid of MRI in a patient referred for the management of TMD. It also underscores the importance of advanced imaging in the diagnosis of unclear orofacial symptoms.
A 67-year-old Caucasian male presented to the Oral Medicine Clinic of the School of Dental Medicine, University of Pennsylvania, with a history of pain in the left preauricular and facial regions and headaches for 1 year. He also reported progressive trismus that had lasted for 4 months. He denied any form of trauma associated with the onset of facial pain. His pain was described as persistent dull aching pain aggravated with jaw function, especially talking and mastication. He obtained minimal relief by taking 600-mg Ibuprofen tablets.
The patient did not report any parafunctional habits or previous episodes of jaw lock, despite occasional nontender bilateral clicking of the TMJs. He had not experienced any previous facial numbness or tingling sensations, but had noticed increasing difficulty putting food in his mouth due to the limited opening of the mouth. This resulted in severe weight loss of 6 pounds within a 4-week period. He had recently changed to a liquid protein supplement diet at the time of presentation. Additionally, the patient also reported sinus problems and recurring ear infections of 3 months' duration, with occasional bloodstained mucous discharge from the nose.
Interestingly, the patient had presented to his general dentist at the onset of his facial pain. It was attributed at that time to inflammation around the left maxillary third molar. After the tooth was extracted, he experienced pain relief for only 2 weeks. He was then referred to an otorhinolaryngologist due to the persistent ear pain. Based on a positive bacterial culture, he was treated with antibiotics, but the pain relief only lasted for 3 weeks. The patient's past medical history was positive for paranoid schizophrenia and bipolar disorder, managed by his primary care physician with risperidone. He had smoked at least a pack of cigarettes daily for 40 years. His review of systems was positive for occasional epistaxis and weight loss, but his vital signs were within the normal limits.
The clinical examination revealed an anxious, cachexic patient. Extraorally, no facial swellings or asymmetry was present, the head and neck lymph nodes were not palpable, and an examination of cranial nerves II-XII showed results within the normal limits.
Intraorally, the Mallampati airway score was 4 and the maximum interincisal mouth opening with firm stop was 20 mm. The modified Mallampati airway score includes class 0 (ability to see any part of the epiglottis upon mouth opening), class 1 (soft palate, fauces, uvula, and pillars visible), class 2 (soft palate, fauces, and uvula visible), class 3 (soft palate and base of the uvula visible), and class 4 (soft palate not visible at all).7 Mild left TMJ pain was present, and a soft reciprocal click was observed on palpation. The mandible deviated to the right on opening. The masticatory muscles were mild to moderately tender, but worse on the left side. Other intraoral examinations could not be completed due to the limited mouth opening. At this point, internal derangement of the left TMJ, headache, and myofascial pain were considered in the differential diagnosis, pending imaging studies.
An MRI scan of the bilateral temporomandibular joints (open and close) with T1- and T2-weighted sagittal and coronal images was ordered to ascertain the cause of the trismus. His pain medication was changed to 15 mg of meloxicam (Mobic®) daily pending the result of the MRI. At a follow-up visit, the maximum interincisal opening had decreased to 15 mm and the patient displayed more difficulty speaking clearly. He had also lost 6 additional pounds within a 4-week period.
The MRI findings were consistent with the presence of a bulky mass with similar signal intensity to the adjacent structures in the left nasopharynx (Fig. 1), oropharynx, left soft palate, vomer, and inferior turbinate (Fig. 2). The mass had also expanded to the masticatory muscles (Figs. 3,4,5), and showed involvement of the masticator space extending to the extramucosal spaces of the skull base (Fig. 6). In addition to soft tissue involvement, erosion of the left mandibular condyle and ramus was also noted (Figs. 7 and 8).
The differential diagnosis was expanded to include nasopharyngeal carcinoma, squamous cell carcinoma, extranodal Hodgkin lymphoma, non-Hodgkin lymphoma, and adenocystic carcinoma. Also considered was nasopharyngeal lymphoid hyperplasia, but this was less likely since the patient did not have a history of human immunodeficiency virus infection.
The patient was immediately referred to an ear, nose, and throat surgeon for evaluation and management of the bulky mass. Following tracheostomy, biopsies were obtained by direct laryngoscopy. Additionally, esophagoscopy and open gastrotomy tube placement were carried out to rule out the spread of the mass to the esophagus and to aid with feeding and nutrition, respectively. Histopathology of the left oropharynx revealed an in situ and invasive poorly differentiated squamous cell carcinoma, with lymphatic invasion. Immunostaining studies confirmed a p16-positive human papilloma virus (HPV)-infected carcinoma. Positron emission tomography-computed tomography (Fig. 9) revealed a hypermetabolic mass measuring 5.0×5.0×7.3 cm, centered in the left naso-oropharynx. It also showed cortical destruction of the medial and lateral pterygoid plates and invasion of the left mandibular ramus and angle (maximum standardized uptake value, 19.4). No distant metastasis was observed, and the tumor was staged as IVb (T4bN2bM0). The patient was treated with a radiation dose of 6996 cGy over 33 fractions (212 cGy/fraction) with concurrent cetuximab. The treatment was well tolerated, with no significant complications other than xerostomia that persisted over 7 months of follow-up.
This report describes the incidental finding of an extensive oropharyngeal mass that extended from the oropharynx to the nasopharynx, mandible, masseter, and base of the skull. Diagnostic studies with MRI for the further evaluation of seemingly innocuous trismus and otalgia were highly instrumental in identifying the cause of the patient's protracted symptoms.
Oropharyngeal squamous cell carcinoma (OPSCC) is a cancer of epithelial origin with increasing incidence in recent years.8 It is known to be associated with HPV in 70% of cases.8 HPV is a DNA virus known to encode numerous oncogenes. The most commonly implicated HPV subtype is HPV-16, which accounts for 90% of all HPV-positive OPSCCs. This subtype is associated with the overexpression of p16, a protein and byproduct of the inhibitory expression of retinoblastoma (Rb) by E7 (an early oncoprotein).9
OPSCC is common in middle-aged Caucasian males in the fourth and fifth decades of life, as exemplified by this patient. It is more prevalent in non-smokers, although smoking can increase the risk of OPSCC, as well as certain sexual behaviors and the use of marijuana.3 The common intraoral sites of presentation are either the base of the tongue or areas hidden within the tonsils. Interestingly, the base of the tongue was spared in this patient. Unfortunately, OPSCC is often asymptomatic at early stages, and only becomes symptomatic as the tumor enlarges and invade adjacent structures. In some cases, otalgia may be the only presenting symptom.10
Histologic examination of biopsied HPV-16 OPSCC tissue usually reveals poorly differentiated non-keratinous epithelium at the basal layer with infiltration of lymphocytes into the connective tissue. This is unlike HPV-negative OPSCC, which is well differentiated and keratinized.1112
The treatment options for HPV-16 OPSCC include surgery, radiation, and chemotherapy. The use of less toxic treatment options is often recommended in the management of HPV p16-positive OPSCC because the patients are younger and display a better 5-year survival rate than patients with HPV-negative OPSCC.1314
As demonstrated in this patient, it is not uncommon for OPSCC to masquerade as TMD due to the unclear symptoms of dull TMJ pain, trismus, deviation on jaw opening, headaches, earaches, and tender or non-tender TMJ clicks and pain during mastication.151516 Other possible symptoms include sinusitis, ear infections, blocked hearing or absolute hearing loss, abnormal cranial nerve examination findings, and epistaxis or nasal obstruction, which further complicate the diagnosis without definitive diagnostic aids such as imaging.1718
A review by Reiter et al. of patients with nasopharyngeal carcinoma with TMD-like symptoms2 revealed that the most common presenting symptoms were trismus, ear involvement, and deviation of the mandible to the contralateral side during mouth opening, as displayed by our patient. The authors also attributed speech difficulty to tumor infiltration of the soft palate. The diagnosis of neoplasms presenting as TMD is still often missed in the early stages of the tumor. Therefore, it cannot be overemphasized that if a patient presents with symptoms incommensurate with the clinical findings,12 it is imperative to request additional advanced radiographic imaging to rule out a neoplasm. The possibility that the pain was caused by a lesion was initially missed by other healthcare providers previously contacted by the patient. This is a clear example of the judicious use of advanced diagnostic imaging, specifically MRI, to make the definitive diagnosis of the cause of ambiguous protracted orofacial pain symptoms.
Figures and Tables
Fig. 1
T1-weighted axial magnetic resonance imaging shows a mass in the left posterior wall of the nasopharynx, with deviation of the nasal septum to the right.
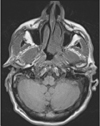
Fig. 2
T1-weighted axial magnetic resonance imaging shows a mass in the left nasopharyngeal wall, left soft palate, vomer, and choana, and inseparable from the inferior turbinate.
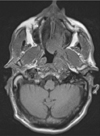
Fig. 3
T1-weighted axial magnetic resonance imaging shows the extension of abnormal soft tissue to the left parapharyngeal space, with no separation from the left masticator space and muscles, specifically the pterygoid and masseter muscles. In addition, extension of the mass to the skull base is present.
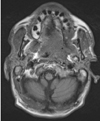
Fig. 4
T1-weighted axial magnetic resonance imaging shows evidence of erosion of the lingual cortex of the left mandibular ramus and presence of abnormal soft tissue in the left retromolar trigone.
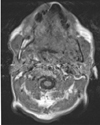
Fig. 5
Enhanced T1-weighted axial magnetic resonance imaging shows the presence of abnormal soft tissue in the left retromolar area.
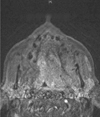
Fig. 6
Nonenhanced T1-weighted coronal magnetic resonance imaging shows the involvement of the pharyngeal area on the left side, with extension to the base of the brain.
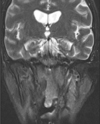
Fig. 7
T1-weighted axial magnetic resonance imaging shows erosive changes on the superficial surface of the left condylar head.
References
1. Grosskopf CC, Kuperstein AS, O'Malley BW Jr, Sollecito TP. Parapharyngeal space tumors: another consideration for otalgia and temporomandibular disorders. Head Neck. 2013; 35:E153–E156.

2. Reiter S, Gavish A, Emodi-Perlman A, Eli I. Nasopharyngeal carcinoma mimicking a temporomandibular disorder: a case report. J Orofac Pain. 2006; 20:74–81.
3. Lewis A, Kang R, Levine A, Maghami E. The new face of head and neck cancer: the HPV epidemic. Oncology (Williston Park). 2015; 29:616–626.
4. Omolehinwa TT, Musbah T, Desai B, O'Malley BW Jr, Stoopler ET. Neuralgia associated with transcutaneous electrical nerve stimulation therapy in a patient initially diagnosed with temporomandibular disorder. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015; 119:e101–e104.

5. Sari-Rieger A, Hassfeld S, Junker K, Rustemeyer J. Adenoid cystic carcinoma of the skull base mimicking temporomandibular disorder. Oral Maxillofac Surg. 2014; 18:115–118.

6. Schneider A, Forstner R. The value of MRI in imaging malignant head and neck tumours. Imaging Decis MRI. 2007; 11:3–10.

7. Lee A, Fan LT, Gin T, Karmakar MK, Ngan Kee WD. A systematic review (meta-analysis) of the accuracy of the Mallampati tests to predict the difficult airway. Anesth Analg. 2006; 102:1867–1878.

8. Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011; 29:4294–4301.

9. Lewis JS Jr, Thorstad WL, Chernock RD, Haughey BH, Yip JH, Zhang Q, et al. p16 positive oropharyngeal squamous cell carcinoma: an entity with a favorable prognosis regardless of tumor HPV status. Am J Surg Pathol. 2010; 34:1088–1096.
10. Chen RC, Khorsandi AS, Shatzkes DR, Holliday RA. The radiology of referred otalgia. AJNR Am J Neuroradiol. 2009; 30:1817–1823.

11. Pitiyage G, Lei M, Guererro Urbano T, Odell E, Thavaraj S. Biphenotypic human papillomavirus-associated head and neck squamous cell carcinoma: a report of two cases. Diagn Pathol. 2015; 10:97.

12. Hennessey PT, Westra WH, Califano JA. Human papillomavirus and head and neck squamous cell carcinoma: recent evidence and clinical implications. J Dent Res. 2009; 88:300–306.

13. Deschler DG, Richmon JD, Khariwala SS, Ferris RL, Wang MB. The “new” head and neck cancer patient-young, nonsmoker, nondrinker, and HPV positive: evaluation. Otolaryngol Head Neck Surg. 2014; 151:375–380.
14. Lorincz BB, Jowett N, Knecht R. Decision management in transoral robotic surgery: indications, individual patient selection, and role in the multidisciplinary treatment for head and neck cancer from a European perspective. Head Neck. 2016; 38:Suppl 1. E2190–E2196.

15. Agulnik M, Epstein JB. Nasopharyngeal carcinoma: current management, future directions and dental implications. Oral Oncol. 2008; 44:617–627.

16. Fischer DJ, Klasser GD, Epstein JB. Cancer and orofacial pain. Oral Maxillofac Surg Clin North Am. 2008; 20:287–301.

18. Khan J, Quek SY, Markman S. Nasopharyngeal carcinoma masquerading as TMJ orofacial pain. Quintessence Int. 2010; 41:387–389.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download