Abstract
Fibrous dysplasia (FD) is an uncommon skeletal disorder in which normal bone is replaced by abnormal fibro-osseous tissue. Mainly, FD is found in children, and by adulthood it usually becomes quiescent. Our case showed FD of more than 14-year duration in the left maxilla. Our evaluation was that growth ceased in adulthood and had achieved the static stage. Because FD cases in elderly patients are rarely reported, we hereby present a monostotic FD case in a 65-year-old female. We presented sequential radiographic images and scintigraphic images of this case, and combined them with a literature review that emphasized the progression of the disease.
Fibrous dysplasia (FD) is a non-malignant condition caused by post-zygotic, activating mutations of the GNAS gene that result in inhibition of the differentiation and proliferation of bone-forming stromal cells and leads to the replacement of normal bone and marrow by fibrous tissue and woven bone.1 The disease may present in a monostotic or polyostotic form, affecting one or multiple bones, respectively. Mainly, FD is found in children, and it usually becomes dormant by adulthood.2 The age range of patients with FD has been reported to be between 20 and 70, with the majority diagnosed in their 30s.3
Despite recent advances in the understanding of the natural history and molecular abnormalities of FD, many questions remain regarding its progression and management. 4
Although most cases of FD are self-limiting5 and hamartomatous, some cases of FD do not go into dormancy at the end of adolescence6 and may be activated or reactivated in adulthood in response to a life event, such as pregnancy.78
Because some cases of FD in elderly people have also been reported,91011 we directed attention to the question of whether a long-term case could show continuous progress or would cease in the quiescent stage. We also examined the incidence of FD transformation into malignancy.
We report here a case of monostotic FD with long duration, with radiographic and scintigraphic images, combined with a literature review that emphasizes the progression of FD.
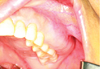
A 65-year-old female visited Wonkwang Dental University Hospital for the removal of a bony dysplastic lesion in the left maxilla. This lesion has been regularly examined in follow-up for the last 14 years. On clinical examination, the left maxilla was enlarged and the bone was hard on palpation. The mucosa overlying the lesion seemed to be intact (Fig. 1). In 2002, she visited our dental hospital for treatment of a periodontal condition on the entire dentition. She had already recognized bony swelling on the left maxilla decades ago, but recalled no experience of pain or fever. Her medical history did not contain anything of significance. Computed tomography in 2002 showed a ground-glass appearance of the left maxilla, which was compatible with FD (Fig. 2). Bucco-lingual cortical expansion, but no cortical destruction, was observed. This lesion extended from the midline to the left maxillary tuberosity. We tentatively diagnosed FD. We recommended removal surgery if pain or discomfort persisted. She refused surgical treatment due to a fear of surgery and felt no need for it, since there was no pain and the lesion was not increasing in size. She preferred watchful waiting. She was advised to visit the dental hospital regularly to check changes in growth. Panoramic radiography in 2007 (Fig. 3) showed a diffuse, widespread, radiopaque lesion in the left maxilla and maxillary sinus. A periapical view in 2007 showed a ground-glass appearance and indistinct anterior border of the lesion. There was no root resorption or displacement of surrounding teeth. The loss of alveolar lamina dura was also observed (Fig. 4).
In 2008, we performed bone scintigraphy using 99mTc-methylene diphosphonate (99mTc-MDP) for detecting the distribution of the lesion. A whole-body bone scan showed intense uptake of radioisotope in the left maxilla (Fig. 5A). Figure 5B shows mild uptake of radioisotope in the vascular and blood pool phase, and intense uptake in the delayed bone scan. Blood pool images were acquired immediately after the flow portion of the study and completed within 10 minutes of tracer injection, for approximately 3.5 minutes/image. Routine delayed images are usually obtained 2-5 hours after injection.
The panoramic view (Fig. 6A, 2016) showed a similar image of the lesion, compared with older images (Fig. 6B, 2012; Fig. 6C, 2008). The cone-beam computed tomography view (Figs. 7A and B) in 2016 showed a similar image and no change in size when compared with older images (Figs. 7C and D) from 2012 and 2002 (Fig. 2). A ground-glass appearance, expanded cortex, no tooth displacement, and slight involvement of the maxillary sinus were common findings. The lesion size was almost 11.8mm×5.2 mm. The lesion seems to have been static for 14 years.
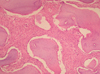
Excision of the FD was carried out and a surgical specimen was sent for histopathological examination. Microscopic examination of the excised specimen showed irregularly shaped woven bone with peritrabecular clefting within a cellular fibrous stroma. The lesional bone fused with the normal cortical bone without a capsule. The trabecular woven bone revealed a lack of osteoblastic rimming and lamellation (Fig. 8). These microscopic features were consistent with FD.
It has been reported that FD represents approximately 5% to 7% of all benign bone tumors.12 Occurrences of FD present in 3 forms that are monostotic (including craniofacial), polyostotic, and polyostotic with endocrinopathies. The monostotic form accounts for 80% to 85% of cases with FD,7 and is not a precursor of the polyostotic form.13 The occurrence of FD is found predominantly in children and young adults, with 75% of patients presenting before the age of 30.1415 Cases in female patients have been observed more frequently in their later decades.16 Monostotic FD of the jaws more commonly involves the maxilla than the mandible,1617 but another study has reported that the mandible was more frequently involved.8 Due to the gradual and painless nature of FD, the clinical findings are usually asymptomatic. If the FD extends into sphenoid, orbital, or frontal bones, complications may include proptosis, visual disturbances, and hearing loss.18 In this case, the patient presented with monostotic FD that was localized to the left side of the maxilla, which caused alveolar bone expansion and facial asymmetry.
The natural progression of FD includes 2 phases: an active phase, which persists until puberty, and a subsequent phase.1920
Many reports have shown that FD progresses slowly or ceases after bone maturation. However, the range in the literature is wide - in some cases it does not progress after puberty,921 while in other cases it continues to progress in old age.2223 Polyostotic FD is more likely to continue to progress even after puberty.12
The present report is focused on FD manifesting in an elderly female patient, which is rare. This case might have been a slow growing lesion in the beginning, which then entered into a quiescent stage in adulthood. We postulated that the patient might not have noticed the severity of this lesion until middle age.
Remodeling of bone to normal lamellar type does not happen in the dysplastic bone of FD. Thus, this bone is likely to be vulnerable to stimulation, which may reactivate it.7 Some cases of reactivation in later stages of life have also been reported.1011 The possible mechanisms of reactivation are trauma, hormonal changes, trauma-included accident, surgery, or tooth extraction.24 Another study of FD that activated or reactivated in pregnant women proposed that female sex hormones are important in the high recurrence rate of FD of the jaws for female patients.725
Some previous studies have reported that there is a very small risk for malignant transformation.26 The risk for malignant transformation is estimated at less than 1% in the monostotic form and at maximally 4% to 6.7% in the polyostotic form;2728 this is less prevalent in patients who have not undergone radiation therapy.28
The current gold standard for the diagnosis of FD is a histologically proven fibro-osseous lesion with poorly defined margins, and definite diagnosis relies on radiographic findings.7 The abnormal trabeculae are usually shorter, thinner, irregularly shaped, and more numerous than normal trabeculae. These abnormal trabeculae and the homogenous radiodensity account for the “ground-glass” appearance in FD.32930 FD affecting the maxilla almost always involves the antrum, either by obliterating it or by gross displacement of its borders.8 The FD extension in this case was directed horizontally, but also slightly vertically. In this growth direction the floor of the left maxillary sinus appeared slightly occupied. The loss of lamina dura was unique to FD and could be used as another diagnostic feature for FD.31
Bone scintigraphy, using 99mTc-MDP is exquisitely sensitive for detecting FD lesions.32 It is a useful imaging modality for detecting the distribution of lesions, and scintigraphy has been evaluated for discriminating between monostotic and polyostotic disease, ruling out metastasis of secondary sarcoma, and recurrence after therapy.33 Monostotic FD, especially in the bones of the skull, generally appears as an area of markedly increased uptake reflecting hyperemia as well as osteoid matrix, with a particularly intense homogenous uptake pattern in the delayed phase.34 The bone scintigraphy in this study showed intense uptake of delayed bone phase although perfusion and blood pool phases showed mild uptake. We think intense uptake of delayed bone phase is due to the increased surface area of fibrous-degenerative bone, rather than the increased vascularity and osteoblast activity of the lesion. That accounts for the quiescent nature of this lesion over its long duration.
Our case showed FD in the left maxilla of a more than 14-year duration with almost the same size. This report emphasizes a case of a hamartomatous nature, involving the quiescent stage of FD in an elderly patient. Our evaluation is that this case ceased to grow in early adulthood, when skeletal maturity had been reached. This diagnosis was supported by sequential radiographic images and scintigraphy. A literature review on the disease activity of FD suggests that reactivation of FD could be possible due to many factors, but most cases remain in the quiescent stage, and malignant transformation is very rare in monostotic FD.
Figures and Tables
Fig. 2
Computed tomography images of axial (A), coronal (B), and sagittal (C) views (2002) show a lesion of homogeneous radiopacity on the left maxilla, with expanded cortex but no cortical destruction.

Fig. 3
A panoramic image (2007) shows gross radiopacity from the midline to left maxillary tuberosity, and the left maxillary sinus in the left maxilla.
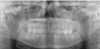
Fig. 4
A periapical radiograph (2007) shows indistinct anterior border of the lesion. Loss of alveolar lamina dura is also seen. The trabeculae seem to be shorter, irregularly shaped, and more numerous than normal trabeculae.
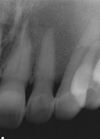
Fig. 5
A. A whole-body bone scan (2008) shows intense uptake of radioisotope in the left maxilla. There is focal increased uptake in the lesser trochanter of the left femur that was supposed to be enthesopathy. B. Mild uptake of radioisotope in the vascular and blood pool phase and intense uptake in delayed bone scan.
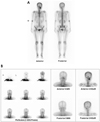
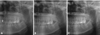
Fig. 6
A cropped panoramic radiograph (A, 2016) shows a similar image of the lesion in the left maxilla, compared with the cropped panoramic view B (2012) and C (2008).
References
1. Lee JS, FitzGibbon EJ, Chen YR, Kim HJ, Lustig LR, Akintoye SO, et al. Clinical guidelines for the management of craniofacial fibrous dysplasia. Orphanet J Rare Dis. 2012; 7:Suppl 1. S2.

2. Mardekian SK, Tuluc M. Malignant sarcomatous transformation of fibrous dysplasia. Head Neck Pathol. 2015; 9:100–103.

3. Tehranzadeh J, Fung Y, Donohue M, Anavim A, Pribram HW. Computed tomography of Paget disease of the skull versus fibrous dysplasia. Skeletal Radiol. 1998; 27:664–672.

4. Ricalde P, Magliocca KR, Lee JS. Craniofacial fibrous dysplasia. Oral Maxillofac Surg Clin North Am. 2012; 24:427–441.

5. Kramer IR, Pindborg JJ, Shear M. The WHO Histological Typing of Odontogenic Tumours. A commentary on the Second Edition. Cancer. 1992; 70:2988–2994.
6. Davies ML, Macpherson P. Fibrous dysplasia of the skull: disease activity in relation to age. Br J Radiol. 1991; 64:576–579.

7. MacDonald-Jankowski D. Fibrous dysplasia: a systematic review. Dentomaxillofac Radiol. 2009; 38:196–215.

8. Nityasri V, Haris PS, Bose T, Balan A. Fibrous dysplasia - a 13-year retrospective radiographic analysis in a south Indian population. Dentomaxillofac Radiol. 2011; 40:282–289.
9. Proschek D, Orler R, Stauffer E, Heini P. Monostotic fibrous dysplasia of the spine: report of a case involving a cervical vertebra. Arch Orthop Trauma Surg. 2007; 127:75–79.

10. Lee SH, Han IH, Kang DW, Choi BK. Cervical fibrous dysplasia presenting as a pathologic fracture in an older patient. J Korean Neurosurg Soc. 2011; 50:139–142.

11. Sachdeva SK. Craniofacial fibrous dysplasia in an elderly patient: a case report with a review of literature. Acta Stomatol Croat. 2015; 49:60–64.

12. DiCaprio MR, Enneking WF. Fibrous dysplasia. Pathophysiology, evaluation, and treatment. J Bone Joint Surg Am. 2005; 87:1848–1864.
13. Lustig LR, Holliday MJ, McCarthy EF, Nager GT. Fibrous dysplasia involving the skull base and temporal bone. Arch Otolaryngol Head Neck Surg. 2001; 127:1239–1247.

15. Ajdinovic B, Jaukovic L, Antoniou D. Five benign myoskeletal diseases in paediatrics and the role of nuclear medicine. Do they differ from those in adults? Hell J Nucl Med. 2013; 16:2–8.
16. MacDonald-Jankowski D. Fibrous dysplasia in the jaws of a Hong-Kong population: radiographic presentation and systematic review. Dentomaxillofac Radiol. 1999; 28:195–202.

17. Singer SR, Mupparapu M, Rinaggio J. Clinical and radiographic features of chronic monostotic fibrous dysplasia of the mandible. J Can Dent Assoc. 2004; 70:548–552.
18. Li J, Li H, Liu X, Han Z. Surgical treatment of polyostotic craniomaxillofacial fibrous dysplasia in adult: a case report and review of the literature. Int J Clin Exp Med. 2015; 8:16756–16764.
20. Mladina R, Manojlovic S, Markov-Glavas D, Heinrich Z. Isolated unilateral fibrous dysplasia of the sphenoid sinus. Ann Otol Rhinol Laryngol. 1999; 108:1181–1184.
21. Ozek C, Gundogan H, Bilkay U, Tokat C, Gurler T, Songur E. Craniomaxillofacial fibrous dysplasia. J Craniofac Surg. 2002; 13:382–389.

22. Olmsted WW. Some skeletogenic lesions with common calvarial manifestations. Radiol Clin North Am. 1981; 19:703–713.
23. Delilbasi C, Deniz E, Ekici ID. Monostotic fibrous dysplasia of the mandible. Oral Health Dent Manag. 2014; 13:326–329.
24. Yetiser S, Gonul E, Tosun F, Tasar M, Hidir Y. Monostotic craniofacial fibrous dysplasia: the Turkish experience. J Craniofac Surg. 2006; 17:62–67.

26. Kim GT, Lee JK, Choi BJ, Kim J, Han SH, Kwon YD. Malignant transformation of monostotic fibrous dysplasia in the mandible. J Craniofac Surg. 2010; 21:601–603.

28. Ruggieri P, Sim FH, Bond JR, Unni KK. Malignancies in fibrous dysplasia. Cancer. 1994; 73:1411–1424.

29. Daffner RH, Kirks DR, Gehweiler JA Jr, Heaston DK. Computed tomography of fibrous dysplasia. AJR Am J Roentgenol. 1982; 139:943–948.

30. Tokano H, Sugimoto T, Noguchi Y, Kitamura K. Sequential computed tomography images demonstrating characteristic changes in fibrous dysplasia. J Laryngol Otol. 2001; 115:757–759.

31. Petrikowski CG, Pharoah MJ, Lee L, Grace MG. Radiographic differentiation of osteogenic sarcoma, osteomyelitis, and fibrous dysplasia of the jaws. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995; 80:744–750.

33. Qu N, Yao W, Cui X, Zhang H. Malignant transformation in monostotic fibrous dysplasia: clinical features, imaging features, outcomes in 10 patients, and review. Medicine (Baltimore). 2015; 94:e369.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download