Abstract
Purpose
Optical coherence tomography (OCT) has been investigated as a novel diagnostic imaging tool. The utilisation of this equipment has been evaluated through several studies in the field of dentistry. The aim of this preliminary study was to determine through basic experiments the effectiveness of OCT in implant dentistry.
Materials and Methods
To assess detection ability, we captured OCT images of implants in each of the following situations: (1) implants covered with mucosae of various thicknesses that were harvested from the mandibles of pigs; (2) implants installed in the mandibles of pigs; and (3) implants with abutments and crowns fixed with temporary cement. The OCT images were captured before cementation, after cementation, and after removing the excess submucosal cement.
Results
If the thickness of the mucosa covering the implant body was less than 1 mm, the images of the implants were clearly detected by OCT. In the implants were installed in pigs' mandibles, it was difficult to capture clear images of the implant and alveolar bone in most of the samples. Remnants of excess cement around the implants were visible in most samples that had a mucosa thickness of less than 3 mm.
Conclusion
Currently, OCT imaging of implants is limited. Cement remnants at the submucosal area can be detected in some cases, which can be helpful in preventing peri-implant diseases. Still, though there are some restrictions to its application, OCT could have potential as an effective diagnostic instrument in the field of implant dentistry as well.
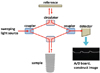
Optical coherence tomography (OCT) is a diagnostic imaging tool that uses low coherence interferometry to determine the delay and magnitude of backscattered light reflected from a biological structure (Fig. 1).12 OCT images differentiate the optical properties of tissues. OCT is an imaging method based on non-ionizing radiation and can provide an image in real-time. The image appears the instant OCT is applied to the object. In the field of ophthalmology, OCT is widely used in the clinic. In the field of dentistry, many studies have been performed for using OCT as a diagnostic tool for dental treatments. Wada et al.3 demonstrated demineralization at non-carious cervical lesions, and Nakagawa et al.4 have shown the correspondence of histological observation and OCT images of carious lesions.
The endosseous dental implant is a well-established prosthetic treatment alternative in modern dentistry.5678 However, as this treatment becomes more common, complications related to dental implants are an increasing problem.9 Of the possible technical or biological complications, peri-implantitis is a major concern because of its lack of an established treatment strategy, its relation to implant loss, and the subsequent difficulty in recovering implant function. Since peri-implantitis is difficult to treat, prevention before the progression of the disease is crucial. For detecting peri-implantitis, probing around an implant and radiographic evaluation are common ways to monitor the peri-implant tissue. With OCT, additional findings could be obtained.
For example, the remnants of luting cement left behind during an implant procedure are known as an iatrogenic cause of peri-implant disease.10 Although the radiograph may disclose the cement left in mesial and distal sites, the cement remnants at buccal and lingual/palatal sites cannot be evaluated without surgical intervention. In such a situation, an OCT image may be helpful for examining the peri-implant tissue without surgical invasion. The aim of this preliminary study was to determine through basic experiments the power of OCT for use in implant dentistry.
A dental OCT system (Prototype 2; Panasonic Healthcare Co., Ltd., Ehime, Japan) was employed in this experiment (Fig. 2). This OCT system incorporates a high-speed frequency-swept external cavity laser with a probe power range from 5 mW to 20 mW. The central wave-length is 1330±10 nm at a 30 kHz sweep rate. The axial resolution of this device is 12 µm in air. The lateral resolution of 20 µm is determined by the objective lens of the probe. A 2000×1019 pixel image is obtained simultaneously and generated in a few hundred milliseconds.
In order to evaluate the detectability of the implant under the mucosal tissue, a dental implant (Straumann standard implant RN, Straumann Japan, Tokyo, Japan) was covered with pig's oral mucosa (Fig. 3). The mucosae were harvested from the hard palates of dead pigs for meal consumption. The thicknesses of the mucosae were measured by penetrating a needle with a rubber stop and their thicknesses varied from 0.35 mm to 2.65 mm. An OCT image of the implant neck surrounded by the mucosa was captured. In order to evaluate the detectability of bone surrounding the implant and the implant under bony tissue, implants were embedded in the jawbone of a dead pig and the OCT image of the marginal parts was captured (Fig. 4). A cone-beam computed tomographic image of this implant is also included in Figure 5.
In order to assess the remnants of luting cement left inside the implant sulcus, an abutment (RN solid abutment, 4-mm height, Straumann Japan, Tokyo, Japan) was fixed to the implants in the pig jaw and a ready-made resin cap (RN Protective Cap for RN Solid abutment, Straumann Japan, Tokyo, Japan) was used as a crown. The cap was connected to the abutment without cement and OCT images were captured. Next, the cap was fixed with temporary cement (Temporary Hard Cement, Shofu, Kyoto, Japan) to the abutment and OCT images were captured after removing supramucosal excess cement. After that, excess cement in the submucosal region was removed using a dental explorer and OCT images were captured. Finally, the mucosa was removed to confirm if there were remnants of cement around the prosthetic margin. The experimental flow is shown in Figure 6. The samples were classified into the following 5 categories: (A) Cements were detected both by OCT and directly; (B) Cements remained but were not detected by OCT (false negative); (C) Cements were detected by OCT, but none remained (false positive); (D) Cements were detected neither by OCT nor directly, and (E) Undeterminable. The thickness and sulcus depth of the peri-implant mucosa in each of the samples were measured and the mean value for each was calculated.
The OCT images displaying the thickness of the pig's oral mucosa are shown in Figure 7. Of the implant samples covered with a mucosa thickness ranging from 0.35 mm to 1.11 mm, the surface of the implant body underneath the mucosa was revealed. However, the object was impossible to detect in samples with a thickness of 2.65 mm.
The captured images of implants embedded in the pig's jawbone are shown in Figure 8. In all images, the implant body and the alveolar bone could not be clearly recognized and only the surface of the mucosa was shown.
Among the 16 samples, the number of each group was as follows: (A) 6; (B) 2; (C) 5; (D) 2; and (E) 1. The range and mean value of the thicknesses of peri-implant mucosae in each group were as follows; (A) 1.55-3.1, 2.24; (B) 2.96-3.3, 3.13; (C) 0.8-3.22, 2.28; (D) 2.5-3.98, 3.24; and (E) 2.3, 2.3. The depths of the peri-implant sulci were as follows: (A) 1-2, 1.67; (B) 2.5-4, 3.25; (C) 1.5-3, 2; (D) 2-3, 2.5; and (E) -1.5, -1.5. Overall, the remnants of cement were detectable when the sulcus depth was less than 2 mm and the thickness of the mucosa was less than 3 mm. The results are summarized in Table 1. OCT images of a typical sample are shown in Figure 9.
In this preliminary study, we investigated the effectiveness of OCT for evaluating peri-implant tissue and detecting foreign bodies at the submucosal area. These could be captured by OCT if the peri-implant mucosa was relatively thin. In most of the other articles, the effective penetrating depth of OCT was 2-3 mm1112 and that coincided with the results of this experiment.
Radiography has been the major diagnostic imaging tool used in dental practice. On the other hand, OCT is entirely different from radiographic imaging, so this might allow us to reveal peri-implant conditions that have never been detected without OCT. In fact, it has been proven that OCT has the potential to evaluate small caries, 4 tooth demineralisation,3 and oral cancer1213 from a different perspective. Each OCT image depicts the highly detailed microstructural images of the surface of the object as if the histologic image were in black and white.
From the results of Experiment 1, the submucosal objects could be detected by OCT if the thickness of the mucosa was less than 2.5 mm, and in the results of Experiment 3, cement remnants at the submucosa surrounding the implant could be detected by OCT as long as the sulcus depth was less than 2 mm and the thickness of the mucosa was less than 3 mm. When it comes to the sulcus depth, the cement might be detected by OCT if the prosthetic margin is appropriately designed, because it is said that to completely remove the cement residue, the margin should be placed less than 1.5 mm submucosally.14
As dental implants increase in number, the complications presented by peri-implant disease have turned into a significant challenge in recent years. In the case of peri-implantitis, as there is still no definitive treatment strategy, early detection and prevention is especially critical. At the same time, iatrogenic factors, such as cement remnants, should be absolutely avoided. Utilization of OCT as an additional modality for monitoring peri-implant tissue may prove significantly beneficial to implant treatments.
Since OCT uses infrared light to depict the object, it is not able to deeply penetrate a red substance such as mucosal tissue and the translucent tissue such as enamel is better for targeting. To dispense with this shortcoming, local anaesthesia with vasoconstrictors might be applied to fade the red color out and make it easy to penetrate. The present experiments sought to demonstrate the detectability of an object visualized using OCT. Therefore, the digitized data of an OCT image may detect the signal changes and structure underneath. Future in vivo studies are required to confirm the efficacy of OCT in clinical practice.
Although OCT imaging of implants is relatively limited at present, structures underneath the peri-implant mucosa, such as left-behind remnants of cement, can be detected in some cases. Additionally, OCT is helpful in preventing peri-implant disease. Despite limitations to its application, OCT has the potential to be an effective diagnostic device in the future.
Figures and Tables
 | Fig. 2A photograph of the machine of a dental optical coherence tomography system (Prototype 2; Panasonic Healthcare Co., Ltd., Ehime, Japan). |
 | Fig. 3A dental implant (Straumann standard implant RN) is covered with pig's oral mucosa for evaluating the detectability of an implant underneath mucosa via optical coherence tomography. |
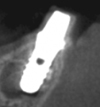 | Fig. 5A cross-sectional cone-beam computed tomographic image shows the embedded implant in the jawbone of the pig. |
 | Fig. 7The optical coherence tomography images of implants surrounded by mucosa in each thickness are lined up. |
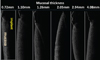 | Fig. 8The optical coherence tomography images of implants embedded in pig's jawbone are lined up with each mucosal thickness. |
References
1. Fujimoto JG. Optical coherence tomography for ultrahigh resolution in vivo imaging. Nat Biotechnol. 2003; 21:1361–1367.

2. Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science. 1991; 254:1178–1181.

3. Wada I, Shimada Y, Ikeda M, Sadr A, Nakashima S, Tagami J, et al. Clinical assessment of non carious cervical lesion using swept-source optical coherence tomography. J Biophotonics. 2015; 8:846–854.

4. Nakagawa H, Sadr A, Shimada Y, Tagami J, Sumi Y. Validation of swept source optical coherence tomography (SS-OCT) for the diagnosis of smooth surface caries in vitro. J Dent. 2013; 41:80–89.

5. Albrektsson T, Brånemark PI, Hansson HA, Lindström J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand. 1981; 52:155–170.
6. Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986; 1:11–25.
7. van Steenberghe D, Lekholm U, Bolender C, Folmer T, Henry P, Herrmann I, et al. Applicability of osseointegrated oral implants in the rehabilitation of partial edentulism: a prospective multicenter study on 558 fixtures. Int J Oral Maxillofac Implants. 1990; 5:272–281.
8. Buser D, Mericske-Stern R, Bernard JP, Behneke A, Behneke N, Hirt HP, et al. Long-term evaluation of non-submerged ITI implants. Part 1: 8-year life table analysis of a prospective multi-center study with 2359 implants. Clin Oral Implants Res. 1997; 8:161–172.

9. Heitz-Mayfield LJ, Needleman I, Salvi GE, Pjetursson BE. Consensus statements and clinical recommendations for prevention and management of biologic and technical implant complications. Int J Oral Maxillofac Implants. 2014; 29:Suppl. 346–350.

10. Lang NP, Berglundh T. Periimplant diseases: where are we now? - Consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol. 2011; 38:Suppl 11. 178–181.

11. Pan Y, Lavelle JP, Bastacky SI, Meyers S, Pirtskhalaishvili G, Zeidel ML, et al. Detection of tumorigenesis in rat bladders with optical coherence tomography. Med Phys. 2001; 28:2432–2440.

12. Chen SF, Lu CW, Tsai MT, Wang YM, Yang C, Chiang CP. Oral cancer diagnosis with optical coherence tomography. Conf Proc IEEE Eng Med Biol Soc. 2005; 7:7227–7229.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download