Abstract
Numerous cases of enamel renal syndrome have been previously reported. Various terms, such as enamel renal syndrome, amelogenesis imperfecta and gingival fibromatosis syndrome, and enamel-renal-gingival syndrome, have been used for patients presenting with the dental phenotype characteristic of this condition, nephrocalcinosis or nephrolithiasis, and gingival findings. This report describes a case of amelogenesis imperfecta of the enamel agenesis variety with nephrolithiasis in a 21-year-old male patient who complained of small teeth. The imaging modalities employed were conventional radiography, cone-beam computed tomography, and renal sonography. Such cases are first encountered by dentists, as other organ or metabolic diseases are generally hidden. Hence, cases of amelogenesis imperfecta should be subjected to advanced diagnostic modalities, incorporating both dental and medical criteria, in order to facilitate comprehensive long-term management.
Amelogenesis imperfecta is characterized by inherited defects in the enamel that can be qualitative or quantitative. The primary dentition, permanent dentition, or both can be affected by this disorder. It may be associated with morphological or biochemical changes elsewhere in the body.1 The kidneys are the most commonly affected organ, and may present with disorders such as nephrocalcinosis, nephrolithiasis, and other functional abnormalities.2 The first case was reported by MacGibbon in 1972;3 multiple cases have been subsequently diagnosed and the condition has been referred to as enamel renal syndrome, amelogenesis imperfecta and nephrocalcinosis, amelogenesis imperfecta and gingival fibromatosis syndrome, or enamel renal gingival syndrome.45 According to a recent review article, only 16 cases have been reported with the complete oral, dental, and renal phenotype that is associated with enamel renal syndrome.4 Although the prevalence of amelogenesis imperfecta is known, the prevalence of enamel renal syndrome has not been assessed, with the exception of one study that emphasized its rarity.6 Affected patients generally suffer from functional and aesthetic problems, leading to an inferior quality of life.7 The severity of the associated renal disorders has been described as ranging from no complications to chronic renal failure.89
A 21-year-old male patient presented with a chief complaint of small upper and lower permanent teeth. According to the history reported by the patient, the size of his teeth never changed with age. No contributing family or medical history was identified. The patient did not report any abnormal oral habits. The findings of his general physical examination were normal.
An intraoral soft tissue examination showed the presence of brown pigmented gingiva with a normal contour (Fig. 1A). A hard tissue examination revealed retained deciduous teeth and permanent teeth with atypical findings, as well as multiple decayed teeth (Figs. 1A-E). Many of the permanent teeth were missing, and the erupted teeth were widely spaced, with a yellowish brown discoloration (Fig. 1A). The enamel layer was absent clinically and post-eruption wearing of the teeth was evident (Figs. 1D-F). The upper central incisors exhibited a semi-lunar shape and the molars had flat occlusal surfaces (Figs. 1A, D, and E). Severe attrition resulted in a collapsed bite and reduced vertical dimensions during occlusion (Figs. 1A and E).
The panoramic radiography and cone-beam computed tomography (CBCT) images revealed multiple findings. All of the teeth were affected, especially the posterior teeth (Fig. 2A). Multiple calcification nodules were seen in the pulp chambers of the primary and permanent teeth; these nodules were needle-shaped in the incisors and round in the posterior teeth (Figs. 2A and B). The differential radiodensity expected between the dentine and the enamel was absent (Fig. 2B). Pericoronal radiolucencies delineated by sclerotic margins around non-erupted teeth were significant (Figs. 2A and B). The impacted teeth presented with complete root formation, exhibiting dilacerations and curvature (Fig. 2A). The dental follicles around the unerupted teeth appeared to be hyperplastic (Figs. 2A and B). Extensive crown resorption with partial replacement of the resorbed dentin by globular calcified structures was observed (Fig. 2C).
The impacted permanent canines, second molars, and third molars were observed to have fully formed roots (Fig. 2A). An accessory mental foramen was seen on the right side of the mandible and extensive bone loss was observed around the mesiobuccal root of the left maxillary first molar in the volume-rendered CBCT images (Figs. 2D and E). A provisional diagnosis of amelogenesis imperfecta was made based on the clinical and radiographic findings. The histopathological examination of an extracted deciduous canine using light microscopy confirmed the absence of enamel and the absence of scalloping of the dentinoenamel junction. (Fig. 3A). Hence, based on the Witkop classification,10 the final diagnosis was the enamel agenesis (type 1G) subtype of hypoplastic-type amelogenesis imperfecta, which is an autosomal recessive disorder. The histopathological analysis of specimens obtained during treatment also confirmed the presence of calcified pulpal tissue (Fig. 3B) and dense collagen bundles with abundant vasculature in the gingival tissue (Fig. 3C).
As this type of amelogenesis imperfecta can be associated with other organ and metabolic abnormalities, further investigations were carried out. An ultrasound examination of his kidneys demonstrated multiple hyperechoic foci in the medulla of both the kidneys, suggesting bilateral nephrolithiasis (Figs. 4A and B). A 24-hour urine spectrophotometric analysis revealed very low citrate levels (18.9 mg/24 hours; reference range, 116-924 mg/24 hours). Other biochemical and haematological findings were normal (alkaline phosphatase, 128 U/L; urea, 12.84 mg/dL, blood urea nitrogen, 6 mg/dL; creatinine, 1.33 mg/dL, blood urea nitrogen to creatinine ratio, 4.51; estimated glomerular filtration rate, >60 mL/min/1.73 m; sodium, 136 mmol/L, potassium, 4.0 mmol/L; chloride, 98 mmol/L; bicarbonate, 19 mmol/L; calcium, 9.4 mg/dL; vitamin D3, 34.63 ng/mL; urine albumin, 32.1 mg/L). A physical, microscopic, and chemical examination of a urine sample did not reveal any abnormalities (urine creatinine, 17.51 mg/dL; urine albumin-creatinine ratio, 183.32 mg/g; 24 hours calculated citrate in urine, 18.9 mg/24 hours; urine oxalate, 14.27 mg/24 hours, urinary calcium, 98.7 mg/24 hours; parathyroid hormone, 19.70 pg/mL).
Thus, based on previous findings,4 the diagnosis was modified to enamel renal syndrome with associated amelogenesis imperfecta, nephrolithiasis, and hypocitraturia.
A general dental practitioner may come across many cases of amelogenesis imperfecta; however, it is difficult to ensure an accurate diagnosis due to its complex classification and the likely absence of relevant data from family members.11 The clinical features of amelogenesis imperfecta vary depending on the type.111213 In the hypoplastic type of amelogenesis imperfect, the teeth exhibit a chalky white to dark brown colour, the occlusal surfaces and incisal edges are generally abraded, and occasionally, the complete loss of the enamel is observed. In the hypocalcified form of amelogenesis imperfecta, the enamel exhibits a cheesy consistency and can be easily removed with a sharp explorer. The hypomaturative type of amelogenesis imperfecta is characterized by normal enamel thickness with the appearance of white opaque areas on the incisal surfaces. Another important fact is the association of amelogenesis imperfecta with other features, comprising a systemic disease or syndrome.11 One such entity is known as enamel renal syndrome.4 De la Dure-Molla et al.4 have established an oral diagnosis criteria which includes clinical, radiographic, and microscopic features characteristic of this syndrome, along with the presence of renal abnormalities. Almost all the findings in our case fit these criteria except for few mentioned below. Radiographically, an abnormal pathway of eruption was not evident in the second molars, and thickening of the maxillary or nasal sinus lining was not observed. Microscopically, the cellular cementum layer characteristic of this syndrome could not be seen. The diagnosis of enamel renal syndrome with associated amelogenesis imperfecta, nephrolithiasis and hypocitraturia was made in this case, as most of the criteria were fulfilled, nephrolithiasis was evident in the ultrasound, and hypocitraturia was observed in the urine analysis.
A renal ultrasound is now recommended in cases with such oral findings.14 Abnormalities in the interstitial matrix have been hypothesized to be the causative factor of dystrophic calcification in the kidney and of enamel abnormalities.15 Low urinary citrate levels indicate that stone formation is more likely, because urinary citrate inhibits stone formation by forming soluble complexes with calcium.16 Other relevant findings in such cases are localized aggressive periodontitis, gingival fibromatosis, and soft tissue calcifications, as described by Kantaputra et al.517 Hence, they coined the term enamel-renal-gingival syndrome. In the present case, localized periodontitis was evident, but the clinical findings did not justify a diagnosis of gingival fibromatosis and histopathological analysis did not reveal any soft tissue calcifications. However, the severity of gingival fibromatosis in such cases can vary even within the same mouth.18 Thus, the present case was diagnosed with enamel renal syndrome, which underscores the fact that all such cases may not necessarily present with gingival abnormalities, as has been reported earlier.417 Enamel renal syndrome is a rare autosomal disorder that has been proven to be associated with mutations in the FAM 20A gene.4517 However, the mutation test was not feasible in the present case.
Detecting an accessory mental foramen on the right side of the mandible was relevant, because it is a rare finding.19 It is important to be aware of variations of the mental foramen in order to achieve successful anaesthesia that avoids nerve injury during periodontal or maxillofacial surgeries and dental implant placement.20 It was observed that volume-rendered images were more useful in detecting accessory foramina and localized aggressive periodontitis.
Based on the findings of ultrasonography and other diagnostic modalities, the patient was informed that he had a higher risk of renal stone formation and was referred to a nephrologist for the necessary preventive treatment. Long term follow-up is imperative for patients with this condition, as some patients may eventually suffer from serious renal dysfunction.9
In conclusion, an adequate knowledge of diseases that involve both dental and medical factors is indispensable for making the correct diagnosis and ensuring comprehensive treatment. Volume-rendered images from CBCT scans can be very valuable in detecting certain abnormalities.
Figures and Tables
Fig. 1
A. A frontal view shows pigmented gingiva, the absence of enamel with spacing between the teeth, and a semi-lunar appearance of the maxillary central incisors. A right lateral view (B), left lateral view (C), and maxillary and mandibular occlusal views (D and E) show the presence of decayed teeth, molars with a flat occlusal surface, multiple deciduous teeth, and multiple missing permanent teeth. F. An extraoral photograph shows the evidence of wear of anterior teeth.
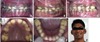
Fig. 2
A. A panoramic radiograph shows pulpal calcifications in most of the teeth (short black arrow), impacted teeth (short white arrows), pericoronal radiolucencies with a sclerotic border (long white arrows), and complete root formation with curvatures (long black arrows). B. A cone-beam CT (CBCT) sagittal image shows uniform radiodensity in the crown (long white arrow) of the dental follicle (long white arrow). C. A CBCT coronal image shows crown resorption (white arrows). D. A CBCT volume-rendered image of the right side of the mandible shows an accessory mental foramen. E. A CBCT volume-rendered image of the left side of the maxilla shows severe bone loss.
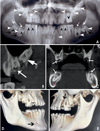
Fig. 3
A. A photomicrograph of a ground section shows the absence of enamel and scalloping of the dentinoenamel junction (black arrows). B. A photomicrograph of the pulpal tissue shows connective tissue with numerous basophilic calcifications (H&E stain, 40×). C. A photomicrograph of the gingival tissue shows parakeratinised epithelium and dense collagen bundles in the lamina propria with fibroblasts and blood vessels (H&E stain, 40×).

Acknowledgements
We thank Dr. Rashmi Gubbi, Dr. Arpan Shah and all other staff members of the Department of Oral and Maxillofacial Pathology and Microbiology for their support. We would like to extend our sincere gratitude to Dr. Satish Shah and Dr. Sheel Shah for helping with the imaging modalities.
References
1. Aldred MJ, Savarirayan R, Crawford PJ. Amelogenesis imperfect: a classification and catalogue for the 21st century. Oral Dis. 2003; 9:19–23.
2. Poornima P, Katkade S, Mohamed RN, Mallikarjuna R. Amelogenesis imperfecta with bilateral nephrocalcinosis. BMJ Case Rep. 2013; 2013:bcr2013009370.

4. de la Dure-Molla M, Quentric M, Yamaguti PM, Acevedo AC, Mighell AJ, Vikkula M, et al. Pathognomonic oral profile of Enamel Renal Syndrome (ERS) caused by recessive FAM20A Mutations. Orphanet J Rare Dis. 2014; 9:84.

5. Kantaputra PN, Kaewgahya M, Khemaleelakul U, Dejkhamron P, Sutthimethakorn S, Thongboonkerd V, et al. Enamel-renalgingival syndrome and FAM20A mutations. Am J Med Genet A. 2014; 164A:1–9.
6. Chosack A, Eidelman E, Wisotski I, Cohen T. Amelogenesis imperfecta among Israeli Jews and the description of a new type of local hypoplastic autosomal recessive amelogenesis imperfecta. Oral Surg Oral Med Oral Pathol. 1979; 47:148–156.

7. Hashem A, Kelly A, O'Connell B, O'Sullivan M. Impact of moderate and severe hypodontia and amelogenesis imperfecta on quality of life and self-esteem of adult patients. J Dent. 2013; 41:689–694.

8. Jaureguiberry G, De la Dure-Molla M, Parry D, Quentric M, Himmerkus N, Koike T, et al. Nephrocalcinosis (enamel renal syndrome) caused by autosomal recessive FAM20A mutations. Nephron Physiol. 2012; 122:1–6.
9. Elizabeth J, Lakshmi Priya E, Umadevi KM, Ranganathan K. Amelogenesis imperfecta with renal disease - a report of two cases. J Oral Pathol Med. 2007; 36:625–628.

10. Witkop CJ Jr. Amelogenesis imperfecta, dentinogenesis imperfecta and dentin dysplasia revisited: problems in classification. J Oral Pathol. 1988; 17:547–553.

12. Chamarthi V, Varma BR, Jayanthi M. Amelogenesis imperfecta: a clinician's challenge. J Indian Soc Pedod Prev Dent. 2012; 30:70–73.

13. Chaudhary M, Dixit S, Singh A, Kunte S. Amelogenesis imperfecta: report of a case and review of literature. J Oral Maxillofac Pathol. 2009; 13:70–77.

14. Hunter L, Addy LD, Knox J, Drage N. Is amelogenesis imperfect an indication for renal examination? Int J Paediatr Dent. 2007; 17:62–65.
15. Lubinsky M, Angle C, Marsh PW, Witkop CJ Jr. Syndrome of amelogenesis imperfecta, nephrocalcinosis, impaired renal concentration, and possible abnormality of calcium metabolism. Am J Med Genet. 1985; 20:233–243.

16. Del Valle EE, Spivacow FR, Negri AL. Citrate and renal stones. Medicina (B Aires). 2013; 73:363–368.
17. Kantaputra PN, Bongkochwilawan C, Kaewgahya M, Ohazama A, Kayserili H, Erdem AP, et al. Enamel-Renal-Gingival syndrome, hypodontia, and a novel FAM20A mutation. Am J Med Genet A. 2014; 164A:2124–2128.
18. Martelli-Júnior H, Bonan PR, Dos Santos LA, Santos SM, Cavalcanti MG, Coletta RD. Case reports of a new syndrome associating gingival fibromatosis and dental abnormalities in a consanguineous family. J Periodontol. 2008; 79:1287–1296.

19. Udhaya K, Saraladevi KV, Sridhar J. The morphometric analysis of the mental foramen in adult dry human mandibles: a study on the South Indian population. J Clin Diagn Res. 2013; 7:1547–1551.
20. Pancer B, Garaicoa-Pazmiño C, Bashutski JD. Accessory mandibular foramen during dental implant placement: case report and review of literature. Implant Dent. 2014; 23:116–124.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download