Abstract
Purpose
Lateral pterygoid muscle (LPM) plays an important role in jaw movement and has been implicated in Temporomandibular disorders (TMDs). Migraine has been described as a common symptom in patients with TMDs and may be related to muscle hyperactivity. This study aimed to compare LPM volume in individuals with and without migraine, using segmentation of the LPM in magnetic resonance (MR) imaging of the TMJ.
Materials and Methods
Twenty patients with migraine and 20 volunteers without migraine underwent a clinical examination of the TMJ, according to the Research Diagnostic Criteria for TMDs. MR imaging was performed and the LPM was segmented using the ITK-SNAP 1.4.1 software, which calculates the volume of each segmented structure in voxels per cubic millimeter. The chi-squared test and the Fisher's exact test were used to relate the TMD variables obtained from the MR images and clinical examinations to the presence of migraine. Logistic binary regression was used to determine the importance of each factor for predicting the presence of a migraine headache.
Temporomandibular disorders (TMDs) are characterized by pain around the temporomandibular joint (TMJ) and jaw muscles, limited jaw movement, and TMJ sounds.1 Frequently, patients with TMDs also describe symptoms of headache, similar to those reported by patients diagnosed with tension-type or migraine headaches, according to the nosology developed by the International Headache Society.2 The relationship between tension-type headache, which was formerly known as musculoskeletal headache, and myalgia of the masticatory muscles is well known and has been demonstrated in many patients with TMDs. However, only a few studies have shown a significant association between vascular headache or migraine and TMDs, with tenderness of the lateral pterygoid muscle (LPM) and the temporalis muscle commonly found in patients with migraine.3,4,5,6
The LPM makes a singular contribution to jaw movement control by virtue of its attachments to components of the TMJ, such as the capsule, disc, and condyle. The superior lateral pterygoid muscle is primarily attached directly to the disc and the TMJ capsule, contributing to disc stability and positioning, whereas the inferior lateral pterygoid muscle contributes primarily to jaw opening.7,8,9
No studies have yet been published relating the volume of the LPM to migraine in patients with TMDs. The purpose of this study was to compare the volume of the LPM in patients with migraine and a control group made up of volunteers without migraine, using the segmentation of the LPM in magnetic resonance (MR) images of the TMJ to determine the volume of the LPM. In addition, the most frequent signs and symptoms of TMDs were investigated in patients with migraine.
Research ethics committee approval was obtained for this study (protocol number 055/2005). The study sample comprised 20 patients with migraine (8 males and 12 females, age range 21-49 years, mean 39.3 years) and the control group comprised 20 volunteers without migraine or any signs or symptoms of TMDs (10 males and 10 females, age range 18-75 years, mean 42.4 years). Patients with migraine were diagnosed and selected by an expert neurologist according to the 2004 International Classification of Headache. Patients with no symptoms of migraine according to the international classification were excluded from the patient group.
Both the control and patient groups underwent a clinical examination of the TMJ conducted by an experienced orofacial pain specialist according to the Research Diagnostic Criteria for TMDs.10 The signs and symptoms of TMDs, such as mandibular range of motion (opening, laterotrusion, and protusion), joint noise, and muscle and/or TMJ pain on palpation, were evaluated and the resulting data were collected. All patients had some signs and symptoms of TMDs in both TMJs for at least five years and were referred by their dentists to the Orofacial Pain and Temporomandibular Disorders Center in the Department of Oral and Maxillofacial Surgery at the State University of Campinas (UNICAMP). Patients taking any type of medication for hypertension or heart disease, patients taking muscle relaxants, and patients who had undergone surgical treatment of the TMJ, orthognathic surgery, or orthodontic treatment were excluded from the sample.
MR imaging was performed on all patients and controls using a 1.5-T MR Imager (Sigma; General Electric, Milwaukee, WI, USA) with a bilateral and dedicated circular polarized 8.0-cm transmit-and-receive TMJ coil. Images were obtained in closed-mouth and open-mouth positions. Using the axial localizer image, parasagittal images (perpendicular to the condylar axis) were obtained and selected to analyze the disc-condyle relationship, using the following protocol.
In the closed-mouth images, the disc was considered to be in normal position when the posterior band of the disc was located at the most superior part of the condyle, as indicated by being in the 12 o'clock position relative to the condyle. The disc was considered displaced when the posterior band of the disc was located anterior to the superior part of the condyle.10 In the open-mouth images, the disc was considered reduced only if it was located between the condyle and the eminence.10
According to this protocol, if the disc was displaced - including anterior, anterolateral, and anterolateral displacements - in the closed-mouth images, and reduced in the corresponding open-mouth images, the disc was classified as experiencing disc displacement with reduction. In contrast, if the disc was displaced in the closed-mouth images and not reduced, but still displaced, in the corresponding open-mouth images, the disc was classified as experiencing disc displacement without reduction.
Condylar motion was considered normal in open-mouth images when the vertex of the condyle was situated beyond the articular eminence, and was considered abnormal when a bite block was necessary to maintain the open-mouth position and avoid motion artifacts.10
The same dentomaxillofacial radiologist evaluated all images, using Merge eFilm Workstation 1.5 (Merge Healthcare, Chicago, IL, USA). The images were interpreted on a 17-inch LCD monitor under appropriate conditions with suitable brightness.
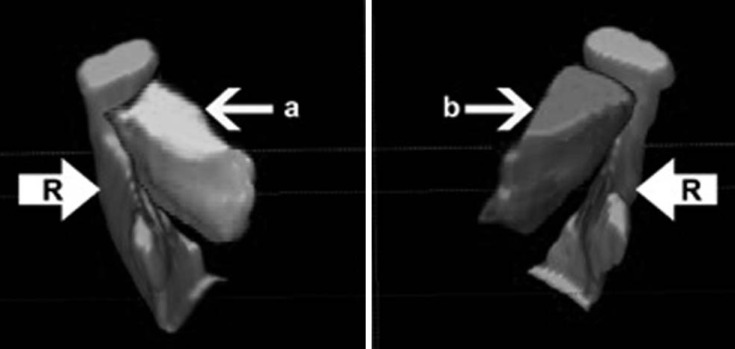
All the closed-mouth images were recorded in the Digital Imaging and Communications in Medicine format and then converted to the Analyze (Mayo Clinic, Rochester, USA) format using the MRIcro software (www.mccauslandcenter.sc.edu, MacCausland Center for Brain Imaging, Columbia, SC, USA). The Analyze format is required for processing and image fusion programs. The DICOM format contains text information about the patient, while the Analyze format only contains the raw image. All the Analyze images were exported into the ITK-SNAP 1.4.1 software (University of North Carolina, Chapel Hill, NC), which calculates the volume of each segmented structure in voxels per cubic millmeter.11 In this study, the images of the muscles in each corresponding slice were segmented in the axial, sagittal, and coronal planes to obtain the LPM volume of each TMJ. The right and left LPMs were segmented individually, using different labels and colors (red for the right and dark pink for the left), in order to obtain the volume for each LPM. The condyle, coronoid process, and part of the ramus of the mandible (labeled in light blue) were also segmented, in order to provide orientation landmarks in the three-dimensional models (Fig. 1).
All the LPM volumes (right and left, separately) were recorded in tables and related to the clinical findings (signs and symptoms) that were obtained by clinical examinations following the Research Diagnostic Criteria for TMDs and by MR imaging of the TMJ. This was carried out in the same manner for all patients with migraine and the control group.
The chi-squared test and the Fisher's exact test, with p-values <0.05 considered to indicate statistical significance, were performed to relate the TMD variables on the MR images to the variables obtained from clinical examinations (disc displacement, disc reduction, normal or abnormal condyle motion, articular and/or muscle pain, mandibular range of motion, and joint noise) for the control and migraine groups. The t-test was used to compare LPM volume values with different variances (P (t=1) one-tailed=0.000001). All tests were performed using the Minitab statistical software (Minitab Inc., State College, PA, USA).
Logistic binary regression was applied in order to study the importance of each factor for predicting the presence of migraine headache.
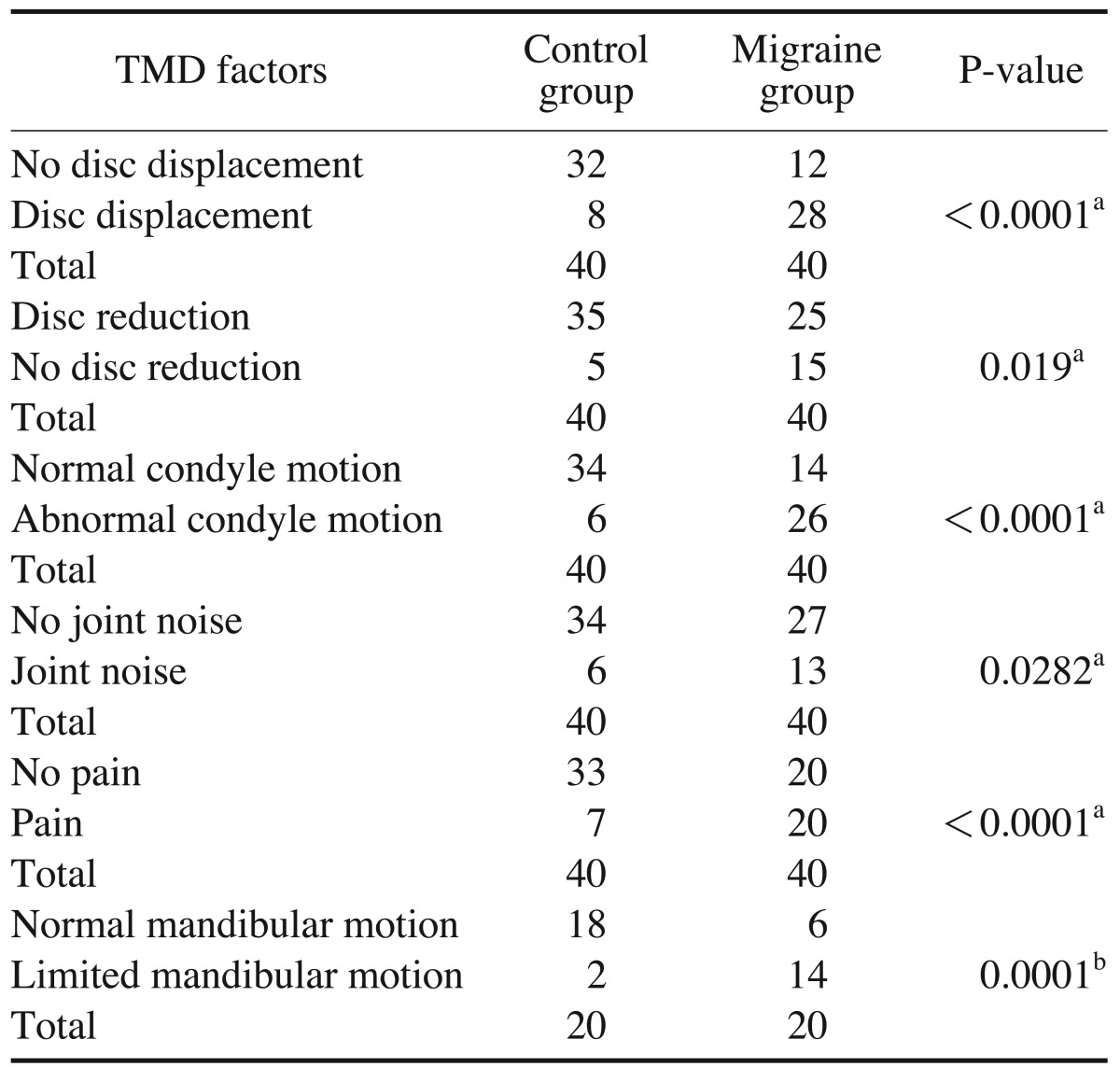
Statistically significant differences between the two groups were found for all the variables analyzed. This was confirmed by the chi-squared and Fisher's exact tests, with p-values <0.05 considered to indicate statistical significance. Disc displacement, absence of disc reduction, abnormal condyle motion, joint noise, articular and/or muscle pain, and limited mandibular range of motion were found more frequently in the migraine group than the control group (Table 1).
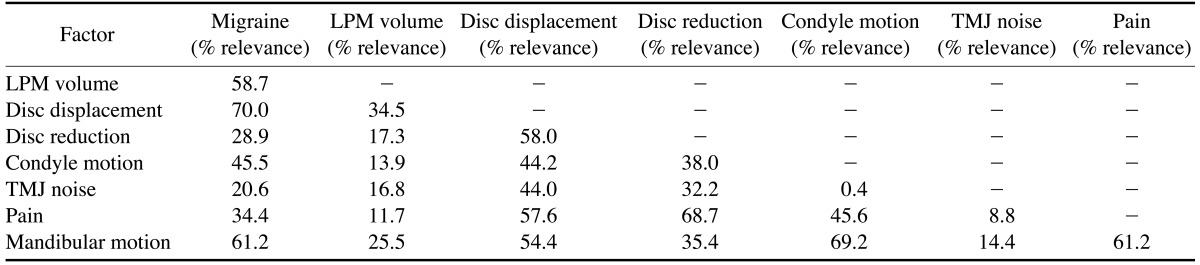
With regard to the volume of the LPM, the t-test showed a statistically significant relationship between migraine and increase of LPM volume. According to logistic binary regression, the relevant factors that predicted the presence of migraine headache were limited mandibular motion (relevance 61.2%), increased volume of the LPM (relevance 58.7%), and disc displacement (70.0%) (Table 2).
Patients diagnosed with painful TMDs often report headaches and pain, typically located in the mastication muscles.3 Although it has been suggested that TMDs can be a trigger for migraine, the role of muscle hyperactivity in migraine has not been clearly established.12 The main results of this study are that the LPM was hypertrophic in most of the patients with migraine and that these patients had signs and symptoms of TMDs (such as pain, joint noise, and disc displacement) when migraine was present. The human LPM plays an important role in the control of jaw movement and has been implicated in TMDs because it is very tender in patients with TMDs.11,13,14 Moreover, a widespread hypothesis exists that abnormal patterns of LPM activity in some patients with TMDs, associated with hyperactivity of the superior head of the LPM, could lead to degenerative arthritic changes in the TMJ.1,13,14 Our study was performed using a specific method that allowed a realistic evaluation of the structure of the LPM, using MR imaging and reliable software to calculate its volume, which was then related to the signs and symptoms of TMDs and the presence of migraine. Previous studies evaluating the functional properties of the LPM in patients with TMDs were based on palpation and electromyographic activity, which is widely known to be a challenging method to apply to this group of muscles.1,13
Another important finding in this study was the relative importance of each factor (TMD signs and symptoms) and the volume of the LPM for predicting the presence or absence of migraine. The results showed that the three most important variables related to migraine in patients with migraine were abnormal mandibular motion, hypertrophic LPM volume, and the presence of disc displacement in the TMJ.10 Our study found a relationship between disc displacement/reduction and abnormal mandibular motion. Mandibular deviation was related to disc displacement with reduction, whereas mandibular deflection was related to disc displacement without reduction.
Considering the high percentage of patients with migraine and disc displacement (70.0%), it is possible that the activity of the LPM can increase in order to stabilize the displaced disc during mandibular movements, which could lead to hypertrophic muscles, as have been observed in patients with TMDs and migraine. Previous studies in which the TMJ was dissected have shown that the superior surface of the upper head of the lateral pterygoid and the midmedial muscle bundle were attached to the disc.15 These findings could explain the hypertrophic LPM that is so common in patients with migraine who have one of the most prevalent signs of disc displacement; abnormal disc position could stimulate LPM activity in an attempt to preserve normal function and position.
D'Ippolito et al.16 studied the visibility of the LPM on MR imaging and concluded that alterations in the thickness of the LPM are more frequent in patients with TMDs.16 In our study, both groups (controls and patients with migraine) had TMDs, but the thickness of the LPM was only significant in the migraine group. Some studies have shown no statistically significant correlations between LPM alterations and TMJ abnormalities; however, they only used visual analysis of the MR images instead of LPM segmentation. We consider the segmentation method to be more accurate than visual analysis, because it provides a numerical value for the volume of the LPM.17
Taskaya-Yılmaz et al.9 concluded that the LPM is atrophied and degenerated in TMJs with disc displacement and no reduction. This result differs from ours, which can probably be explained by their visual evaluation of atrophy and degeneration on MR images, compared to our use of specific software to accurately calculate the real segmented volume of the LPM. In addition, the duration of the symptoms may help to explain the difference between their findings and ours. In our study, the patients with migraine had acute symptoms, which can increase the volume of the LPM. However, Taskaya-Yılmaz et al.'s study contained no information about whether the symptoms were acute or chronic, and the decreased LPM volume they observed was probably related to the presence of chronic symptoms in their patients.
It may prove useful to consider the inferior and superior parts of the LPM separately. Some studies have examined the muscle architecture of the superior and inferior heads of the LPM using cadaveric specimens.18 We did not perform segmentation separately on the superior and inferior parts of the LPM in order to consider the distinct heads of the muscle, because it was difficult to determine the real limits of each head on the MR images. Moreover, this method could lead to misinterpretation and erroneous conclusions about the LPM.
In conclusion, this study showed that the LPM tends to be hypertrophic in patients with TMDs and simultaneous migraine. Furthermore, disc displacement and abnormal mandibular movements seem to be the most common signs in patients with migraine and TMDs. Although not all patients with TMDs and migraine showed LPM hypertrophy, our results suggest that TMDs can be a cause of LPM hypertrophy. Moreover, our results suggest that the known difficulties in using palpation alone to study the LPM may be circumvented by employing LPM segmentation on MR imaging as an alternative method for studying this muscle group.
References
1. Murray GM, Bhutada M, Peck CC, Phanachet I, Sae-Lee D, Whittle T. The human lateral pterygoid muscle. Arch Oral Biol. 2007; 52:377–380. PMID: 17141177.

2. Lipton RB, Bigal ME, Steiner TJ. Classification of primary headaches. Neurology. 2004; 63:427–435. PMID: 15304572.

3. Ballegaard V, Thede-Schmidt-Hansen P, Svensson P, Jensen R. Are headache and temporomandibular disorders related? A blinded study. Cephalalgia. 2008; 28:832–841. PMID: 18498400.

4. DeRossi S, Stoopler E, Sollecito T. Temporomandibular disorders and migraine headaches: comorbid conditions? Internet J Dent Sci. 2005; 2.
5. Svensson P. Muscle pain in the head: overlap between temporomandibular disorders and tension-type headaches. Curr Opin Neurol. 2007; 20:320–325. PMID: 17495627.

6. Kreisberg MK. Headache as a symptom of craniomandibular disorders. I: Pathophysiology. Cranio. 1986; 4:135–142. PMID: 3458835.

7. Murray GM, Phanachet I, Uchida S, Whittle T. The role of the human lateral pterygoid muscle in the control of horizontal jaw movements. J Orofac Pain. 2001; 15:279–292. PMID: 12400398.
8. Murray GM, Phanachet I, Uchida S, Whittle T. The human lateral pterygoid muscle: a review of some experimental aspects and possible clinical relevance. Aust Dent J. 2004; 49:2–8. PMID: 15104127.

9. Taskaya-Yilmaz N, Ceylan G, Incesu L, Muglali M. A possible etiology of the internal derangement of the temporomandibular joint based on the MRI observations of the lateral pterygoid muscle. Surg Radiol Anat. 2005; 27:19–24. PMID: 15750717.

10. Tasaki MM, Westesson PL. Temporomandibular joint: diagnostic accuracy with sagittal and coronal MR imaging. Radiology. 1993; 186:723–729. PMID: 8430181.

11. Wang MQ, Yan CY, Yuan YP. Is the superior belly of the lateral pterygoid primarily a stabilizer? An EMG study. J Oral Rehabil. 2001; 28:507–510. PMID: 11422675.

12. Lamey PJ, Burnett CA, Fartash L, Clifford TJ, McGovern JM. Migraine and masticatory muscle volume, bite force, and craniofacial morphology. Headache. 2001; 41:49–56. PMID: 11168603.

13. Türp JC, Minagi S. Palpation of the lateral pterygoid region in TMD - where is the evidence? J Dent. 2001; 29:475–483. PMID: 11809325.
14. Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006; 31:1116–1128. PMID: 16545965.

15. Headache Classification Subcommittee of the International Headache Society. The international classification of headache disorders. 2nd ed. Oxford: Blackwell Pub;2004. p. 9–160.
16. D'Ippolito SM, Borri Wolosker AM, D'Ippolito G, Herbert de Souza B, Fenyo-Pereira M. Evaluation of the lateral pterygoid muscle using magnetic resonance imaging. Dentomaxillofac Radiol. 2010; 39:494–500. PMID: 21062943.
17. Dergin G, Kilic C, Gozneli R, Yildirim D, Garip H, Moroglu S. Evaluating the correlation between the lateral pterygoid muscle attachment type and internal derangement of the temporomandibular joint with an emphasis on MR imaging findings. J Craniomaxillofac Surg. 2012; 40:459–463. PMID: 21880502.

18. Davies JC, Charles M, Cantelmi D, Liebgott B, Ravichandiran M, Ravichandiran K, et al. Lateral pterygoid muscle: a threedimensional analysis of neuromuscular partitioning. Clin Anat. 2012; 25:576–583. PMID: 22144260.

Fig. 1
Three-dimensional model of the segmented structures using the ITK-SNAP software in an anterior superior view shows the lateral pterygoid muscle (a, b), the condyle, the coronoid process of the mandible, and part of the mandibular ramus (R).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download