Abstract
Primitive neuroectodermal tumor (PNET), which belongs to the Ewing's sarcoma (ES) family of tumors, is mainly seen in children and young adults. PNETs are extremely rare in the maxilla. Here, we report a case of PNET of the left maxillary sinus in an elderly male. Magnetic resonance imaging (MRI) revealed a slightly enhanced solid mass occupying the left maxillary sinus and infiltrating into the retroantral space. A partial maxillectomy was performed. Despite postoperative chemotherapy, follow-up computed tomography (CT) and MRI revealed a nodal metastasis in the submandibular space. Neck dissection was performed. However, the patient died 10 months after the second surgery because of distant metastasis to the liver. MRI and CT were particularly useful in detecting the extent of the tumor, recurrence, and metastasis. Further, a literature review of the previously reported PNET cases of the maxilla was carried out. In this paper, we also discuss the current approach for the diagnosis and management of these tumors.
Primitive neuroectodermal tumor (PNET) is a rare, highly malignant small round cell neoplasm that has poor prognosis. PNET belongs to the Ewing's sarcoma (ES) family of tumors. Both PNET and ES share a common neuroectodermal origin.1,2 However, PNET exhibits a more aggressive clinical behavior than ES and metastasizes very rapidly.3,4 The term "PNET" was first introduced by Hart and Earle5 to describe medulloblastoma-like lesions in the cerebral hemisphere. In 1986, Dehner6 suggested differentiation between central and peripheral PNETs. Subsequently, PNET arising from the central nervous system was termed "central PNET (cPNET)", while tumors developing outside the central and autonomic nervous systems were called "peripheral PNETs (pPNETs)".7 PNET accounts for 1%-4% of all soft tissue neoplasms.2,8 It is more common in children and young adults.11 PNET mostly affects the thoracopulmonary region, pelvis, abdominal region, and extremities;2,8,10,11 less frequently, it occurs in the head and neck areas.4,12,13 PNET of the maxilla is exceedingly rare among elderly people. Only 10 cases1,8,10,11,14,15,16,17,18,19 of PNETs of the maxilla have been reported in the literature. Here, we report a case of a 67-year-old male diagnosed with PNET of the maxillary sinus after detailed clinical, radiological, and histopathological examinations.
A 67-year-old man presented at a local dental clinic with pain in the left maxillary second molar tooth. His medical history was unremarkable. He was diagnosed with chronic periodontitis and underwent extraction of the affected tooth. However, the pain continued, and 2 weeks after the extraction, he again presented with the complaint of a non-healing extraction socket. Examination revealed granulation tissue and debris in the extracted socket area with associated gingivobuccal swelling. He underwent a curettage operation. A biopsy specimen was taken. The histopathological report revealed a small focal cluster of round cells and blood clots. The patient was referred to Seoul National University Dental Hospital for further investigation because of a suspicion of malignancy.
At the Seoul National University Dental Hospital, the patient underwent thorough clinical, radiological, and histopathological examinations. Panoramic radiography revealed generalized sclerosis of both maxillary sinuses with increased radiopacity in the left maxillary sinus. Thinning of the innominate line of the zygomatic process of the left maxilla was evident (Fig. 1). Both the panoramic and posteroanterior skull projections showed erosion of the posterolateral wall of the left maxillary sinus (Fig. 2). Magnetic resonance (MR) images revealed a solid mass presumably arising from and occupying the left maxillary sinus with infiltration into the retroantral space posteriorly, and gingivobuccal sulcus inferiorly (Fig. 3). A slightly hyperintense T2 signal and minimal enhancement were noted. A bone scan revealed increased uptake in the left maxilla (Fig. 4).
Histologically, the tumor was composed of sheets of small round to oval cells, which were arranged in lobules, separated by fibrous septa. Most cells were characterized by ill-defined, scanty, pale-staining cytoplasm and well-defined nuclei with coarse chromatin (Fig. 5). Immunohistochemical studies demonstrated positive immunoreactivity for MIC2 (CD99), neuron-specific enolase (NSE), S-100 protein, and cytokeratin (CK) (Fig. 6). The diagnosis of PNET was made on the basis of microscopic and immunohistochemical findings. A partial maxillectomy was performed (Fig. 7). Following the surgery, the patient received one cycle of chemotherapy with anti-cancer drugs vincristine and cisplatin. No additional chemotherapy was given, as the overall condition of the patient was deteriorating. Three months after the surgery, the patient was readmitted with an enlarged left submandibular lymph node. A follow-up computed tomographic (CT) examination revealed a 2-cm mass in the left submandibular space compressing the submandibular gland indicating local nodal metastasis (Fig. 8). The lymph node metastasis exhibited minimal enhancement. The patient was put on a two-cycle chemotherapy treatment. However, there was no response to chemotherapy. A fat-suppressed T2-weighted MR image, taken 4 months after the CT scan, revealed a marked enlargement of the metastatic submandibular lymph node (Fig. 9). Surgery was performed to eliminate the mass in the neck. The tumor was a well-encapsulated friable mass, and its cut surface showed a gray-tan fleshy appearance with focal areas of necrosis and hemorrhage. Microscopically, the tumor cells had small round nuclei that varied from vesicular with inconspicuous nucleoli to stippled chromatin with ill-defined cell borders. There were numerous mitoses. Neither rosettes, pseudorosettes, nor neurotubules were identified (Fig. 10). On the basis of these findings, the tumor was interpreted as metastatic PNET. Following the second surgery, the patient decided not to continue his treatment. Six months later, the patient developed distant metastasis of tumor cells to the liver; he died 10 months after the second operation.
PNET is not a common tumor in the head-neck region. Jürgens et al4 reported 42 cases of PNETs. Of these, only 4 were head-neck PNETs. None of the cases reviewed by Marina et al12 were head-neck PNETs. In their series of 54 cases, Kushner et al13 found only 1 case of head-neck PNET. However, Jones and McGill11 reported that the head-neck region was the second most common site for PNET after the thoracopulmonary region. Incidence of PNETs in the maxilla is even rarer. Of the 11 cases studied by Jones and McGill,11 only 1 arose in the maxilla. Windfuhr1 made an excellent review of 27 cases of head-neck PNETs. In the report, only 6 cases occurred in the maxilla. However, after an extensive search of the existing English-language literature, we could identify 10 reported cases of PNETs of maxilla.1,8,10,11,14,15,16,17,18,19 The clinical presentations, imaging features, microscopic findings, treatment modalities, and clinical courses of these cases are presented in Table 1. PNET has been reported to occur in young people.9 One study1 reported that 85% of head-neck PNET patients were younger than 20 years of age. Of the 10 reported cases of maxillary PNETs that we identified, 6 patients were under the age of 20 years. We did not find any sex predilection in the maxillary PNETs. This finding was consistent with previous reports. Most of the reported cases of PNETs of the maxilla occurred either as a soft tissue mass or swelling (Table 1).
Like those of PNETs of other body parts, radiological findings of head-neck PNETs were non-specific (Table 1).1,8,10,11,14,15,16,17,18,19 On plain radiographs, they appeared as areas of bone destruction. On CT images, the PNETs we found appeared as heterogeneously enhancing masses. On T1-weighted MRI, these tumors have isointense presentation to muscle, whereas on T2-weighted MRI, PNETs show a heterogeneous hyperintense signal. In our case, the relatively low T2-weighted MR signal intensity was indicative of a solid tumor with high cellularity. The central low signal intensity was possibly due to bleeding/hematoma/hemorrhage from the initial curettage/surgical procedure. Initial histopathological reports from the left maxillary sinus also confirmed the presence of blood clot/necrotic tissues. In our opinion, the lesion arose from the mucosal lining of the maxillary sinus. However, we did not rule out the bony area surrounding the maxillary sinus as the possible site of origin. Once developed, the lesion infiltrated the maxillary alveolar bone, which resulted in mobility of the maxillary molar tooth. After extraction of the tooth, the growth accelerated further. Minimal enhancement of the enlarged lymph node on the follow-up enhanced CT image gave the impression of central necrosis. However, T2-weighted MRI demonstrated that there was no necrotic portion of a markedly high signal intensity. The minimal enhancement on the enhanced CT scan might have been caused by the high cellularity of the metastatic lymph node as tumors with high cellularity normally show delayed enhancement or a low degree of enhancement. Ibarburen et al17 studied the CT and MRI findings of 17 patients with peripheral PNET. They reported that both CT and MRI are very useful in preoperative staging and in planning the surgical approach. CT and MRI were also helpful in the detection of recurrent and metastatic disease. We believe that MRI should be considered the imaging modality of choice for pre- and post-surgical assessments of PNET, as it is more effective than CT in detecting soft tissue abnormalities and their relation to adjacent vascular and nervous structures.
Due to their highly unpredictable behavior, the management of PNETs is often challenging. Thus far, no standard treatment protocol has been developed for PNETs.1 The current approach for the management of these tumors includes a combination of therapeutic modalities like early surgery along with multiple chemotherapy to treat the residual disease and to prevent metastatic or recurrent disease.1,2,11,13,20 Multi-agent chemotherapy including vincristine, doxorubicin, and cyclophosphamide improves survival without any significant morbidity.21,22 Zimmermman et al22 reported that chemotherapy is mandatory as the first-stage treatment in order to avoid a mutilating surgical procedure and intraoperative tumor cell dissemination. This treatment may be supplemented by radiotherapy, which should be given to patients who do not have a surgical option that restores physiology and to patients whose tumor has been excised with an inadequate margin.1 Given the particularly aggressive nature of PNET, effective local control requires tumor-free surgical margins and wide safety margins.2 However, such margins may not always be available in the maxilla region because of its proximity to vital structures. In the present case, the proximity of the mass to the skull base prevented us from further resection. In addition, the patient's overall weak condition kept us from an aggressive use of radiation therapy and multi-agent chemotherapy, which presumably accelerated the tumor growth and metastasis.
Although steadily improving, the prognosis of PNET is generally considered to be poor with a high incidence of rapid metastasis to distant sites such as the lung, liver, and bone.1,2 The patient in the present report also developed metastasis to the liver 6 months after the second surgery. Kimber et al20 reported that tumors arising from the head and neck, as well as from the chest, had an intermediate prognosis compared with tumors from the paraspinal and scapular areas, which were associated with a favorable outcome. Jones and McGill11 reported that 27% patients with head-neck PNET had metastatic disease at the time of presentation. In contrast, in the series of Nikitakis et al,2 all the 5 head-neck PNET patients were alive. Only 1 patient developed distant metastasis. Prognosis of PNET mainly depends on tumor location, tumor size, presence of metastatic disease at the time of initial diagnosis, and response to initial chemotherapy. The reported 3-year survival rate is about 50%.2,3,4,8 Survival in the study of Mendel et al23 was an average of 27.3 months for asymptomatic patients and 15.2 months for symptomatic patients from the time of initial diagnosis. In the cases of maxillary PNET we reviewed,1,8,10,11,14,15,16,17,18,19 elderly patients seemed to have a relatively poor prognosis compared with their younger counterparts although only 3 patients had undergone follow-up for 3 years at the time of the study.
PNET is a very aggressive malignancy with a poor survival rate. It should be included in the differential diagnosis of fast-growing soft tumors, particularly those that have imaging features of high cellularity.
Figures and Tables
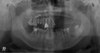
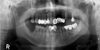 | Fig. 1Panoramic radiograph shows increased radiopacity in the left maxillary sinus with thinning of the innominate line of the zygomatic process of the left maxilla. |
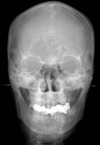 | Fig. 2Posteroanterior skull radiograph demonstrates erosion of the lateral wall of the left maxillary sinus. |
 | Fig. 3Magnetic resonance images reveal hyperintense T2 (A) and hypointense T1 signal lesion (B) with minimal enhancement occupying left maxillary sinus (C, D). Note the infiltration into the retroantral space posteriorly, and gingivobuccal sulcus inferiorly. The central low signal intensity with a dark rim is possibly due to a hematoma from the initial curettage/surgical procedure. |
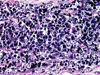
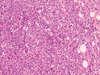 | Fig. 5Photomicrograph of a histopathological slide of primary tumor shows tumor cells with round to oval outlines, arranged in a lobular pattern. The nuclei are well-defined and hyperchromatic, while the cytoplasm is very scanty (H&E stain, 200×). |
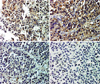 | Fig. 6Photomicrographs A. Tumor cells are stained positively for CD99, (CD99 stain, 200×). B. The majority of tumor cells are positive for neuron-specific enolase (NSE stain, 200×). C. A few tumor cells are positive for S-100 protein (S-100 stain, 200×). D. Tumor cells show a focal positive reaction for cytokeratin (CK stain, 200×). |
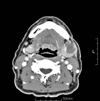 | Fig. 8Follow-up enhanced computed tomography (CT) image, taken 3 months after the operation, reveals a minimally enhancing 2-cm mass in left submandibular space compressing the submandibular gland. |
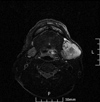 | Fig. 9Follow-up fat-suppressed T2-weighted MR image, taken after 4 months after the follow-up CT scan, shows an increase in size of the metastatic lymph node in the left submandibular space. |
References
1. Windfuhr JP. Primitive neuroectodermal tumor of the head and neck: incidence, diagnosis, and management. Ann Otol Rhinol Laryngol. 2004; 113:533–543.

2. Nikitakis NG, Salama AR, O'Malley BW Jr, Ord RA, Papadimitriou JC. Malignant peripheral primitive neuroectodermal tumor-peripheral neuroepithelioma of the head and neck: a clinicopathologic study of five cases and review of the literature. Head Neck. 2003; 25:488–498.

3. Schmidt D, Herrmann C, Jürgens H, Harms D. Malignant peripheral neuroectodermal tumor and its necessary distinction from Ewing's sarcoma. A report from the Kiel Pediatric Tumor Registry. Cancer. 1991; 68:2251–2259.

4. Jürgens H, Bier V, Harms D, Beck J, Brandeis W, Etspüler G, et al. Malignant peripheral neuroectodermal tumors. A retrospective analysis of 42 patients. Cancer. 1988; 61:349–357.
5. Hart MN, Earle KM. Primitive neuroectodermal tumors of the brain in children. Cancer. 1973; 32:890–897.

6. Dehner LP. Peripheral and central primitive neuroectodermal tumors. A nosologic concept seeking a consensus. Arch Pathol Lab Med. 1986; 110:997–1005.
7. Batsakis JG, Mackay B, el-Naggar AK. Ewing's sarcoma and peripheral primitive neuroectodermal tumor: an interim report. Ann Otol Rhinol Laryngol. 1996; 105:838–843.

8. Mohindra P, Zade B, Basu A, Patil N, Viswanathan S, Bakshi A, et al. Primary PNET of maxilla: an unusual presentation. J Pediatr Hematol Oncol. 2008; 30:474–477.
10. Alobid I, Bernal-Sprekelsen M, Alós L, Benítez P, Traserra J, Mullol J. Peripheral primitive neuroectodermal tumour of the left maxillary sinus. Acta Otolaryngol. 2003; 123:776–778.

11. Jones JE, McGill T. Peripheral primitive neuroectodermal tumors of the head and neck. Arch Otolaryngol Head Neck Surg. 1995; 121:1392–1395.

12. Marina NM, Etcubanas E, Parham DM, Bowman LC, Green A. Peripheral primitive neuroectodermal tumor (peripheral neuroepithelioma) in children. A review of the St. Jude experience and controversies in diagnosis and management. Cancer. 1989; 64:1952–1960.

13. Kushner BH, Hajdu SI, Gulati SC, Erlandson RA, Exelby PR, Lieberman PH. Extracranial primitive neuroectodermal tumors. The Memorial Sloan-Kettering Cancer Center experience. Cancer. 1991; 67:1825–1829.

14. Slootweg PJ, Straks W, Noorman van der Dussen MF. Primitive neuroectodermal tumour of the maxilla. Light microscopy and ultrastructural observations. J Maxillofac Surg. 1983; 11:54–57.
15. Filiatrault D, Jéquier S, Brochu P. Pediatric case of the day. Primitive neuroectodermal tumor (PNET) of the right maxillary sinus. Radiographics. 1993; 13:1397–1399.

16. Shah N, Roychoudhury A, Sarkar C. Primitive neuroectodermal tumor of maxilla in an adult. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995; 80:683–686.

17. Ibarburen C, Haberman JJ, Zerhouni EA. Peripheral primitive neuroectodermal tumors. CT and MRI evaluation. Eur J Radiol. 1996; 21:225–232.

18. Kao SY, Yang J, Yang AH, Chang KW, Chang RC. Peripheral primitive neuroectodermal tumor of the maxillary gingivae with metastasis to cervical lymph nodes: report of a case. J Oral Maxillofac Surg. 2002; 60:821–825.

19. Sun G, Li Z, Li J, Wang C. Peripheral primitive neuroectodermal tumour of the maxilla. Br J Oral Maxillofac Surg. 2007; 45:226–227.

20. Kimber C, Michalski A, Spitz L, Pierro A. Primitive neuroectodermal tumours: anatomic location, extent of surgery, and outcome. J Pediatr Surg. 1998; 33:39–41.

21. Rivera-Luna R, Gomez-Martínez R, Leal-Leal C, Cardenas-Cardos R, Castellanos-Toledo A, Rueda-Franco F, et al. Is the ancillary chemotherapy approach of any value in the treatment of infratentorial primitive neuroectodermal tumors with surgery and radiotherapy. Childs Nerv Syst. 1998; 14:109–112.

22. Zimmermann T, Blütters-Sawatzki R, Flechsenhar K, Padberg WM. Peripheral primitive neuroectodermal tumor: challenge for multimodal treatment. World J Surg. 2001; 25:1367–1372.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download