Abstract
Purpose
The aim of this study was to analyze three-dimensional images of the arterial supply to the temporomandibular joint.
Materials and Methods
Ten patients (five men and five women, mean age 36 years) without signs or symptoms of temporomandibular disorders, who underwent contrast-enhanced computed tomographic (CT) scanning with intravenous contrast, were studied. The direct volume rendering technique of CT images was used, and a data set of images to visualize the vasculature of the human temporomandibular joint in three dimensions was created. After elaboration of the data through post-processing, the arterial supply of the temporomandibular joint was studied.
Results
The analysis revealed the superficial temporal artery, the anterior tympanic artery, the deep temporal artery, the auricular posterior artery, the transverse facial artery, the middle meningeal artery, and the maxillary artery with their branches as the main arterial sources for the lateral and medial temporomandibular joint.
Conclusion
The direct volume rendering technique was found to be successful in the assessment of the arterial supply to the temporomandibular joint. The superficial temporal artery and maxillary artery ran along the lateral and medial sides of the condylar neck, suggesting that these arteries are at increased risk during soft-tissue procedures such as an elective arthroplasty of the temporomandibular joint.
The temporomandibular joint (TMJ) is a diarthrodial joint consisting of the mandibular condyle, squamous portion of the temporal bone, fibrous capsule with reinforcement, and accessory ligaments such as the sphenomandibular, stylomandibular, and pterygomandibular ligaments, and the synovial membrane.1
The blood supply to the TMJ is circumferential. Every vessel within a radius of some three centimeters contributes branches to the joint capsule and contributes one or two branches to it.2 The blood vessels to the TMJ mainly originate from the superficial temporal artery (STA, about 3.8 mm in diameter) and the maxillary artery (MA, about 3.2 mm in diameter).3
The MA is classified according to its relationship with the lateral pterygoid muscle and zygomatic arch. The superficial MA is located laterally to the lateral pterygoid muscle and with a distance between zygomatic arch and maxillary artery of ≥20 mm. The deep MA is medial to the lateral pterygoid and with a distance between zygomatic arch and maxillary artery of >20mm(Fig. 1).4
Other vessels which supply the TMJ have been described: these are small branches of the external carotid artery (ECA, the auricular posterior artery 1.7 mm in diameter and the ascending pharyngeal artery >1 mm in diameter), and of the facial or the ascending palatine artery.3
In the retrodiskal tissue (RT), which is responsible for the nutrition of the TMJ, are present the branches of the maxillary artery (posterior auricular, anterior tympanic (ATA), and meningeal medial arteries) and the temporomandibular veins, as well as the auriculotemporal and posterior auricular nerves.5
We reviewed the cervicocranial arteries of 10 patients without signs or symptoms of temporomandibular disorder (TMD) (five men and five women, age range from 25 to 46 years, mean 36 years) who underwent contrast-enhanced computed tomographic (CT) scanning in the period from October 2008 to January 2009, at Santa Teresa Clinic, Bagheria, Palermo.
A standardized TMD examination was executed in all of the patients: joint pain, crepitation, and uncoordinated movements of the head of the mandibular condyle during opening or closing of the mouth were investigated by lateral and posterior palpation of each TMJ with both index fingers. Subjects were included if they had a temporomandibular index (TMI) reference value of ≤0.08±0.10, and an intensity of jaw pain <5 (with 1 indicating mild pain and 5 moderate pain).12,13
All of the subjects gave written informed consent before beginning the study. The Medical Ethics Committee of the Policlinico G. Martino approved the study protocol, which conformed to the principles of the Declaration of Helsinki for human subject research.
The examinations were performed with a four-channel CT scanner (Mx8000 Quad, Philips Medical Systems, Best, the Netherlands). The volume acquisition was performed during administration of 90 mL of iodinated non-ionic contrast agent at a concentration of 370 mg/mL through an antecubital vein, with an 18-gauge needle cannula using an automatic injector (Envision, Medrad, Pittsburgh, PA, USA) at a flow rate of 4 mL/s. To obtain optimal enhancement of the arteries, the delay time between beginning the contrast agent administration and scan acquisition was calculated with the bolus test technique by measuring the enhancement curve at one of the common carotid arteries. Optimizing arterial enhancement with the bolus test technique, we deliberately overlooked the venous district, thus avoiding possible interference with the images.
The acquisition volume was set in the caudocranial direction from C7 to the sella turcica. The scan parameters were 250 mAs, 120 kV, collimation 40×0.625 mm, pitch 0.67, gantry rotation time 0.5 seconds, and acquisition time 10 seconds. For the rendering process, we used VolView 2.0 graphics software (Kitware Inc. Clifton Park, NY, USA). The direct volume rendering system made use of specific algorithms to transform conventional two-dimensional magnetic resonance imaging sets of slices transparent volume data set images and enabled visualization of data from various imaging modalities (e.g. CT, MRI, functional MRI, and confocal microscopy) interfaced with various hardware and software systems.
The CT images were compared to the anatomic tables. The arterial branch on the CT images was categorized as traceable (2 points), recognizable only for a short trace (1 point), or unrecognizable (0 point). The total score for each artery was divided by 20 and multiplied by 100 to arrive at a depiction rate for each artery.14
Applying volume rendering techniques, the three-dimensional images of the temporomandibular region were obtained. Shading features were used to simulate an external light source. This was performed to give additional depth cues and surface texture to the image, offering a more realistic view of the anatomic structures.
In particular, the use of these procedures permitted visualization of bone and vascular structures contemporarily. In this way, the arteries and their branches were identified, based on a comparison with anatomical tables of the human TMJ.3,15-17
In this study, most of the patients demonstrated the following features. Observing the temporomandibular region posteriorly (Fig. 1), it was possible to find the ECA (a) curving somewhat anteriorly and then inclining dorsally to the space behind the neck of the mandible, where it divided into the IMA (b) and STA(c); from the STA arose small diverging arteries (e). In this image, it was possible to identify the transverse facial artery (TFA), a branch of the STA, which disappeared into the parotid gland (d) and the middle meningeal artery (f), a branch of the mandibular portion of the MA.
Rotating the image and then observing the left TMJ (Fig. 2), it was possible to find the same vascular organization. In addition, on this side, the inferior dental artery (IDA, f), a branch of the mandibular portion of the IMA, was visible until it disappeared behind the mandibular ramus and the anterior tympanic artery (ATA, h), branch of the IMA, which passed behind the TMJ to enter the tympanic cavity.
Modifying the parameters and magnifying the region of the mandibular condyle, it was possible to better observe the relations of these vessels with bone structures (Fig. 3). In particular, the ECA (a), the IMA (b), and the TFA (c), with their small diverging branches (d), a small portion of the IDA (e), the middle meningeal artery (MMA, f), and finally the ATA(g) were observed.
Removing the ramus of the mandible, all of the vessels of the region behind the ramus were visible. In particular, the entire extension of the IMA was observable with its branches (Fig. 4A). Here, the IMA (b), IDA (e), MMA (f), ATA (g), masseteric artery (MA), pterygoid artery (PA), sphenopalatine artery (SA), and deep temporal artery (DTA) were found. A schema of the IMA, with its branches, was also identified in order to demonstrate the fidelity of our study by 3D volume rendering (Figs. 4 and 5).
By observation of our image, it was clear that most of the vascular supply appeared to come from the lateral and medial aspects of the condylar head. The posterior disc attachment region was greatly vascularized, while the intermediate zone and the anterior disc attachment region were relatively devoid of vessels.
Besides the ECA, the arterial supply to the retrodiscal tissue (RT), the STA, the IMA, the IDA, and the MMA were seen in all cases (depiction rates all 100%). The TFA, the MA, the ATA, and the condylar branches of the STA were found in several but not all cases (depiction rates 70%, 100%, 60%, and 50%, respectively). The analysis of the depiction rates showed that the direct volume rendering technique consistently depicted larger arteries and the zone with a higher degree of vascular supply, but also arteries with a thin diameter (Table 1).
The knowledge of the blood supply around the TMJ is necessary in order to clarify how the blood supply works in the pathogenesis of TMD. Three-dimensional volume rendering has been used for evaluation of other arteries such as the cervicocranial arteries, intracranial aneurysms, and pulmonary artery.18-20 However, these arteries have a larger size than those of the TMJ.
This study showed that three-dimensional volume rendering of computed tomography angiography can successfully be used to delineate the vascular anatomy of the TMJ region in good detail.
By this technique, it could be observed that the lateral and ventral region of the articulation was mainly vascularized by the STA and its branches. The anterior region was vascularized by the posterior DTA, the medial region by the ATA and medial meningeal arteries.21,22
The study by Wasicky and Pretterklieber revealed that while the left ATA originated as a singular vessel from either the MA or the STA with almost equal frequencies (44.7 and 45.9%, respectively), the right ATA predominantly branched from the MA (77.8% of cases).23 Mérida Velasco et al, in 18 adult cadavers, described the ATA as one of the main arteries of the RT and found that it is an ever-present artery.11 Even though Takagi et al found that MR angiography depicted the ATA in only 25% of patients, probably because the artery was very thin in caliber, in our study, the three-dimensional volume rendering of computed tomography angiography depicted the ATA in 60% of patients.6
The circulatory systems of the TMJ can be compromised by trauma, disease, changes of the head and neck position, and muscle spasm.
Surgery of the TMJ (disc repair procedures, menisectomy with implant, condylotomy, condylectomy, arthroscopy, and other procedures) is an effective treatment for structural disorders, and there are several general surgical indications (documented refractory internal derangements, pain and dysfunction of such magnitude). Some of these procedures require open surgery with full mandibular condyle exposure via large skin incisions (disc repair, menisectomy, bone reduction procedures), and some are performed percutaneously, with access to the mandibular condyle via multiple limited skin incisions (arthroscopy). The bleeding and vascular injuries associated with trauma to the carotid artery and its terminal branches, the STA, and the IMA are common complications.24
There have been a few reported cases of injury to the MA during intraoral vertical ramus osteotomies, subcondylar and condylar fractures, and TMJ surgery.25 Those cases were associated with aggressive surgical techniques and necessitated ligation of the ECA to control hemorrhage.
Holmlund and Hellsing demonstrated the close proximity of an arthroscopy puncture site to the STA and vein.26 The close proximity of the MMA to the medial capsule could increase the risk of hemorrhage during TMJ surgery (for example, in eminectomy, condylectomy, or during release of joint ankylosis).27 Branches of the IMA may be damaged during extensive maxillary fractures (Le Fort I and II) or repair.
The retroarticular region is very important to cushion from mechanical stress and to protect the tympanic wall. A posterior condylar position, often related to the anterior displacement of the joint disk, reduces the posterior intraarticular space and represents a compression on the bilaminar zone.28,29
The symptomatology in this scenario can be expressed as pain of TMJ, limited movement or locking of the jaw, radiating pain in the face, neck or shoulders, painful clicking, popping, or grating sounds in the jaw joint when opening or closing the mouth, tinnitus, vertigo, and otalgia. Heffez and Jordan found a statistically significant association between superficial vascular changes (avascularity) in the RT and progressive anterior displacement of the TMJ disk.30
In the head and neck region, arteriovenous malformations and arteriovenous fistulas are uncommon but pose serious therapeutic challenges. Most of the acquired arteriovenous fistula cases involve the internal carotid artery and are more often secondary to blunt, penetrating injury and surgical procedures. The development of an arteriovenous fistula between the MA and the venous malformation has sometimes been reported after Le Fort I osteotomy.31 Pseudoaneurysm and arteriovenous malformation of the STA and vein have also been described after arthroscopy.32
Aneurysms of the head vessels must be recognized as potential complications in the long-term follow-up of incidental trauma and iatrogenic injury. The branches of the ECA are protected from trauma in most regions of the head and neck by bone and soft tissue. However, in specific areas, the vessels emerge from their protective buffer, consequently leaving them particularly vulnerable to traumatic injury. Such areas are encountered as the vessels approach the surface and cross bony structures. For example, the STA traverses the zygomatic arch after it exits from the parotid gland, leaving the artery relatively unprotected.33
Conner et al revealed that, of the 386 reported cases of traumatic pseudoaneurysm of the face and temple, 85% were found in the STA, 7% were in the IMA, and 8% were in the facial artery.34 Cases of pseudoaneurysm of the IMA secondary to trauma have been reported.35,36
STA pseudoaneurysms due to iatrogenic injury have been reported to occur after cyst removal, TMJ excision, or arthroplast.37 The post-traumatic development of an aneurysm can lead to expansion under arterial pressure and potential rupture. Often, there is a delay between the initial injury and the development of the aneurysm, ranging from weeks to years.38
In conclusion, a number of imaging techniques have been used to assess the TMJs. The most prevalent alteration involving the TMJs are dysfunctional conditions (internal derangement) and non-dysfunctional diseases (arthritis, infections, coronoid process hyperplasia, secondary neoplastic process, fractures, synovial chondromatosis, and avascular necrosis of the mandibular condyle).
Computed tomography and magnetic resonance imaging are important in the diagnosis of diseases of this region because they present a higher diagnostic accuracy as compared with conventional radiology, considering their higher anatomical resolution.
The three-dimensional volume rendering of computed tomography angiography is a promising non-invasive diagnostic tool for evaluate the vascular anatomy of the TMJs, and widens the frontiers for the further understanding of TMJ disorders as related to vascular abnormality, in addition to the planning of surgical procedures.
Figures and Tables
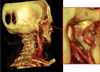 | Fig. 1Posterior view of computerized tomography rendering of the temporomandibular joint of a 30-year-old healthy female. The external carotid artery (a), the internal maxillary artery (b), the superficial temporal artery (c), the transverse facial artery (d), with their small diverging arteries (e), the middle meningeal artery (g), the retrodiscal tissue (i), the ramus (l) and the condyle (m) |
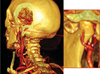
 | Fig. 2Posterior and lateral view of computerized tomography rendering of the temporomandibular joint of a 30-year-old healthy female. The external carotid artery (a), the internal maxillary artery (b), the superficial temporal artery (c), the transverse facial artery (d), the inferior dental artery (f), the middle meningeal artery (g), the anterior tympanic artery (h), the retrodiscal tissue (i), the ramus (l) and the condyle (m), the temporal posterior artery (t) |
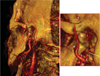
 | Fig. 3Posterior view of computerized tomography rendering of the temporomandibular joint of a 30-year-old healthy female. The external carotid artery (a), the internal maxillary artery (b), the transverse facial artery (d) with their small diverging arteries (e), the inferior dental artery (f), the middle meningeal artery (g), the anterior timpanic artery (h), the retrodiscal tissue (i), the ramus (l) and the condyle (m) |
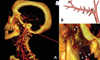 | Fig. 4Computerized tomography rendering of the left temporomandibular joint of a 28-year-old healthy male (A) and diagram (B) show the branches of maxillary artery. The external carotid artery (a), the internal maxillary artery (b), the inferior dental artery (f), the middle meningeal artery (g), the condyle (m), the masseteric artery (n), the pterygoid artery (o), the sphenopalatine artery (p), the sphenopalatine foramen (q), the deep temporal artery (v) |
 | Fig. 5Lateral angiograms (A) and diagrams (B and C) of the internal maxillary artery and the superficial temporal artery arising from the external carotid artery. The external carotid artery (a), the internal maxillary artery (b), the superficial temporal artery (c), the transverse facial artery (d), the inferior dental artery (f), the masseteric artery (n), the pterygoid artery (o), the sphenopalatine artery (p), the occipital artery (r), the auricular posterior artery (s), the temporal posterior artery (t), the deep temporal anterior artery (u), the deep temporal artery (v) |
References
1. Molinari F, Manicone PF, Raffaelli L, Raffaelli R, Pirronti T, Bonomo L. Temporomandibular joint soft-tissue pathology, I: Disc abnormalities. Semin Ultrasound CT MR. 2007. 28:192–204.

2. Patnaik VV, Bala S, Singla RK. Anatomy of temporomandibular joint? A review. J Anat Soc India. 2000. 49:191–197.
3. Ezure H, Mori R, Ito J, Otsuka N. Case of a completely absent facial artery. Int J Anat Var. 2011. 4:72–74.
4. Putz R, Pabst R. Sobotta atlas of human anatomy. Vol. 1: head, neck, upper limb. 2001. 13 rev. ed. Munich: Urban & Fischer;86–87.
5. Hatcher DC, Blom RJ, Baker CG. Temporomandibular joint spatial relationships: osseous and soft tissues. J Prosthet Dent. 1986. 56:344–353.

6. Takagi R, Shimoda T, Westesson PL, Takahashi A, Morris TW, Sano T, et al. Angiography of the temporomandibular joint. Description of an experimental technique with initial results. Oral Surg Oral Med Oral Pathol. 1994. 78:539–543.
7. Piette E, Lametschwandtner A. The angioarchitecture of the rat mandibular joint synovium. Arch Oral Biol. 1995. 40:487–497.

8. Piette E, Lametschwandtner A. The angioarchitecture of the rat mandibular joint bilaminar zone. Arch Oral Biol. 1995. 40:499–505.

9. Piette E, Lametschwandtner A. The fine vasculature of the rat mandibular joint. Acta Anat (Basel). 1995. 153:64–72.

10. Kvinnsland S, Kvinnsland I, Kristiansen AB. Effect of experimental traumatic occlusion on blood flow in the temporomandibular joint of the rat. Acta Odontol Scand. 1993. 51:293–298.

11. Mérida Velasco JR, Rodríguez Vázquez JF, Jiménez Collado J. Anterior tympanic artery: course, ramification and relationship with the temporomandibular joint. Acta Anat (Basel). 1997. 158:222–226.

12. Pehling J, Schiffman E, Look J, Shaefer J, Lenton P, Fricton J. Interexaminer reliability and clinical validity of the temporomandibular index: a new outcome measure for temporomandibular disorders. J Orofac Pain. 2002. 16:296–304.
14. Takagi R, Westesson PL, Ohashi Y, Togashi H. MR angiography of the TMJ in asymptomatic volunteers. Oral Radiol. 1998. 14:69–74.

15. Uysal II, Buyukmumcu M, Dogan NU, Seker M, Ziylan T. Clinical significance of maxillary artery and its branches: a cadaver study and review of the literature. Int J Morphol. 2011. 29:1274–1281.

16. Boyer CC, Williams W, Stevens FH. Blood supply of the temporomandibular joint. J Dent Res. 1964. 43:224–228.

17. Funakoshi K. Nutrient arteries of the temporomandibular joint: an anatomical and a pathological study. Okajimas Folia Anat Jpn. 2001. 78:7–16.
18. Sparacia G, Bencivinni F, Banco A, Sarno C, Bartolotta TV, Lagalla R. Imaging processing for CT angiography of the cervicocranial arteries: evaluation of reformatting technique. Radiol Med. 2007. 112:224–238.

19. Benvenuti L, Chibbaro S, Carnesecchi S, Pulera F, Gagliardi R. Automated three-dimensional volume rendering of helical computed tomographic angiography for aneurysms: an advanced application of neuronavigation technology. Neurosurgery. 2005. 57:1 Suppl. 69–77.

20. Ferretti GR, Arbib F, Bertrand B, Coulomb M. Haemoptysis associated with pulmonary varices: demonstration using computed tomographic angiography. Eur Respir J. 1998. 12:989–992.

21. Godlewski G, Bossy J, Giraudon M, Dussaud J, Pavart JC, Lopez JF. Arterial vascularization of the temporomandibular joint. Bull Assoc Anat (Nancy). 1978. 62:229–236.
22. Siéssere S, Vitti M, Semprini M, Regalo SC, Iyomasa MM, Dias FJ, et al. Macroscopic and microscopic aspects of the temporomandibular joint related to its clinical implication. Micron. 2008. 39:852–858.

23. Wasicky R, Pretterklieber ML. The human anterior tympanic artery. A nutrient artery of the middle ear with highly variable origin. Cells Tissues Organs. 2000. 166:388–394.
24. Cillo JE, Sinn D, Truelson JM. Management of middle meningeal and superficial temporal artery hemorrhage from total temporomandibular joint replacement surgery with a gelatinbased hemostatic agent. J Craniofac Surg. 2005. 16:309–312.

25. Rajab BM, Sarraf AA, Abubaker AO, Laskin DM. Masseteric artery: anatomic location and relationship to the temporomandibular joint area. J Oral Maxillofac Surg. 2009. 67:369–371.

26. Holmlund A, Hellsing G. Arthroscopy of the TMJ. An autopsy study. Int J Oral Surg. 1985. 14:169–175.
27. Talebzadeh N, Rosenstein TP, Pogrel MA. Anatomy of the structures medial to the temporomandibular joint. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999. 88:674–678.

28. Weinberg LA. The etiology, diagnosis, and treatment of TMJ dysfunction-pain syndrome. Part II: Differential diagnosis. J Prosthet Dent. 1980. 43:58–70.

29. Tallents RH, Macher DJ, Kyrkanides S, Katzberg RW, Moss ME. Prevalence of missing posterior teeth and intraarticular temporomandibular disorders. J Prosthet Dent. 2002. 87:45–50.

30. Heffez LB, Jordan SL. Superficial vascularity of temporomandibular joint retrodiskal tissue: an element of the internal derangement process. Cranio. 1992. 10:180–191.

31. Goffinet L, Laure B, Tayeb T, Amado D, Herbreteau D, Arbeille P, et al. An arteriovenous fistula of the maxillary artery as a complication of Le Fort I osteotomy. J Craniomaxillofac Surg. 2010. 38:251–254.

33. Stewart CL, Cohen-Kerem R, Ngan BY, Forte V. Post-traumatic facial artery aneurysm in a child. Int J Pediatr Otorhinolaryngol. 2004. 68:1539–1543.

34. Conner WC 3rd, Rohrich RJ, Pollock RA. Traumatic aneurysms of the face and temple: a patient report and literature review, 1644 to 1998. Ann Plast Surg. 1998. 41:321–326.
35. Bozkurt M, Kapi E, Karakol P, Yorgancilar E. Sudden rupture of the internal maxillary artery causing pseudoaneurysm (mandibular part) secondary to subcondylar mandible fracture. J Craniofac Surg. 2009. 20:1430–1432.

36. Walker MT, Liu BP, Salehi SA, Badve S, Batjer HH. Superficial temporal artery pseudoaneurysm: diagnosis and preoperative planning with CT angiography. AJNR Am J Neuroradiol. 2003. 24:147–150.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download