Abstract
Purpose
This study was performed to obtain a quantitative evaluation of the cortical and cancellous bone graft harvestable from the mental and canine regions, and to evaluate the cortical vestibular thickness.
Materials and Methods
This study collected cone-beam computed tomographic (CBCT) images of 100 Italian patients. The limits of the mental region were established: 5 mm in front of the medial margin of each mental foramen, 5 mm under the apex of each tooth present, and above the inferior mandibular cortex. Cortical and cancellous bone volumes were evaluated using SimPlant software (SimPlant 3-D Pro, Materialize, Leuven, Belgium) tools. In addition, the cortical vestibular thickness (minimal and maximal values) was evaluated in 3 cross-sections corresponding to the right canine tooth (3R), the median section (M), and the left canine tooth (3L).
Results
The cortical volume was 0.71±0.23 mL (0.27-1.96 mL) and the cancellous volume was 2.16±0.76 mL (0.86-6.28 mL). The minimal cortical vestibular thickness was 1.54±0.41 mm (0.61-3.25 mm), and the maximal cortical vestibular thickness was 3.14±0.75mm(1.01-5.83 mm).
Conclusion
The use of the imaging software allowed a patient-specific assessment of mental and canine region bone availability. The proposed evaluation method might help the surgeon in the selection of the donor site by the comparison between bone availability in the donor site and the reconstructive exigency of the recipient site.
Implant rehabilitation necessitates an adequate conformation to the residual alveolar crest, including bone height and width; this condition is often neglected, especially in the case of edentulism, either age-related or subsequent to facial trauma.
Vertical and horizontal resorption makes it difficult to place implants. In the presence of severe bone atrophy, it is possible to restore a correct bone conformation with different techniques such as vascularized and non-vascularized bone grafts, guided bone regeneration, and tissue engineering.1
In oral surgery, crucial attention is paid to graft techniques. Autologous bone, having osteoconductive, osteoinductive, and osteogenic properties (known as the "regenerative triad"), is unanimously considered to be the gold standard in peri-implant bone reconstruction surgery.2
Autologous bone can be collected from intra- or extra-oral sites. For intra-oral sites, the symphysis menti, mandibular ramus, and maxillary tuberosity would be available, and for extra-oral sites, the iliac crest, tibial plateau, rib, and calvaria would be available. The intra-oral sites offer numerous advantages compared to the extra-oral ones, such as easier surgical access, shorter surgical time, absence of cutaneous scarring, and reduced morbidity, but they allow obtaining only limited bone amounts. The selection of the donor site mainly depends on the dimensions of the bone defect. Intra-oral sites are recommended in the presence of small defects, while extra-oral ones are recommended for large defects.3
Oral surgery's requirements are usually fulfilled by intraoral donor sites, with the possibility of harvesting from multiple sites (mental region+mandibular ramus).4 On the other hand, maxillofacial surgery has different requirements, given the fact that it necessarily involves the traumarelated lack of maxillary continuity due to a shooting weapon, accidents, and so on; wide head and neck reconstruction after oncology surgery; or serious malformation deficits.
Bone grafts from intra-oral donor sites are on average more successful than those from extra-oral sites. This fact suggests that site-specific differences may influence the graft integration process.5 Embryonic development and the complex osteocartilaginous interaction of the craniofacial skeleton provide evidence for the peculiarity of the osteogenic properties of this bone.6
A recent study evaluated the different activity of the resident multipotent human bone marrow stromal cells (hBMSCs) taken from intra-oral (jaw, mandible) and extraoral (iliac crest) donor sites. The orofacial hBMSCs produced a greater amount of bone in vivo, as they required a lower quantity of factors for the induction of osteogenic differentiation. This makes intra-oral donor sites the first choice for autologous bone graft.5
Among intra-oral donor sites, many studies have suggested choosing, when possible, the mandibular ramus. This is related to post-surgery morbidity: patients are less able to discern neurosensory disturbances in the posterior buccal soft tissues than in the lower lip.7 Patients should be informed about the potential hazard of modified sensitivity in the chin region.8
Nevertheless, the accessibility of the mandibular symphysis area seems to be better than that of the mandibular ramus,9 especially if the patient has limited jaw opening or temporomandibular joint dysfunction.10 Furthermore, the symphysis graft was found to have the largest cancellous component when compared with mandibular ascending ramus/body, coronid process, and zygomatic-maxillary buttress grafts.4
The aim of the present study was to obtain a quantitative evaluation of cortical and cancellous bone grafts harvestable from the mental region for reconstructive surgery, and to evaluate the cortical vestibular thickness.
Cone beam computed tomographic (CBCT) images of patients were retrieved from the datasets of Italian patients of the Sapienza University of Rome Department of Oral Surgery. The CBCT images were screened for image quality, and those with artifacts secondary to patient motion or major dental artifacts were excluded. The CBCT images of the patients who had pathologies such as cysts or tumors in the mandible, osteoporosis, osteomyelitis, or osteopetrosis in the mandible, history or evidence of previous surgery in the mandible, history of maxillofacial trauma, or asymmetry of the mandible were also excluded.
In the end, this study included the CBCT images of 100 patients. The patients comprised 61 females and 39 males, and the patients' ages ranged from 19 to 91 years. Figure 1 shows the patients' age distribution in this study. This study was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2000. All of the patients had given informed consent for the use of their images for educational and scientific purposes. The CBCT images had been acquired using CBCT equipment (GENDEX GXCB-500, Gendex Dental Systems, Des Plaines, IL, USA), and they were acquired from April 2009 to November 2012. The CBCT images were acquired with a 0.2-mm voxel size and an 8 cm × 8 cm field of view (FOV) set at 120 kVp, 5 mA, and 23 s exposure time.
The Digital Imaging and Communications in Medicine (DICOM) files of each CBCT were transferred to SimPlant (SimPlant 3-D Pro; Materialize, Leuven, Belgium) software. The software accepts images in DICOM format, the standard format used for sharing and visualizing all types of medical images. Once acquired with the SimPlant software, the images were elaborated in axial, paraxial, and sagittal sections. The images were real radiographic representations with 1 : 1 reproduction scale of the jaw bones, and the software enabled the measurement of the anatomical structures.
The DICOM images of the mandibular region (230 images on a total of 432 images for each CBCT scan) were selected through the SimPlant "image selector" tool. Then, through the "Segmentation" tool, the 3D reconstruction of the mandibular region was completed by the manual elimination of the "scattering" caused by the dental prosthesis or metallic restorations into the oral cavity. Segmentation allowed for creating a 3D model from the 2D CBCT images and required an appropriate thresholding of the density value. An automatic segmentation of the bone structures within 500 and 3,071 HU has been used. A lower limit of 500 HU was chosen in accordance with Nakano et al.20
The 3D reconstruction image was oriented to have the mandible parallel to the floor. Subsequently, through the "create a panxoramic curve" tool, the scout view of reference was selected and the mean line with cross-sections at 1-mm intervals was reconstructed through the software. At this stage, the "create nerve" tool showing the course of the left and right mandibular canal either on the cross sections or in the 3D reconstructions was used. The "mental region"11,12 was defined as bounded by lines 5 mm in front of the medial margin of each mental foramen for the lateral limit, 5 mm under the apex of each tooth present on the cross-sectional image for the upper limit, and the upper part of the inferior mandibular cortex on the crosssectional image for the lower limit (Fig. 2).
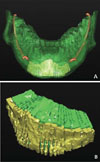
The volume of the cortical and cancellous bone in the mental region was measured using the "graft-volume-create a volume" tool of the software. The area of interest was selected on each cross section with the help of a multilinear mouse pointer. It was selected manually by dragging a pointer along the cortical and cancellous area perimeter. The area values were progressively elaborated by the software to obtain cumulative volumetric measurements (multiplying the selected cortical/cancellous area of each crosssection by 1-mm intervals between the slices). The cortical and cancellous bone volumes were distinguished by using a different chromatic code, which allowed enhanced graphical 2D and 3D representations (Fig. 3A).
Moreover, this software tool automatically provided a mean value of the bone density as the Hounsfield Unit (HU) in the selected cortical and cancellous bone. Next, the cortical vestibular thickness data (linear measurements) were acquired. Three representative cross-sections for each CBCT were selected, including the right canine tooth (3R), the median section of the mandible (M), and the left canine tooth (3L) cross-sections (Fig. 3B). For each of these cross-sections, two data points, the minimal and the maximal buccal cortical thicknesses, were acquired (Fig. 3C).
Figure 4 demonstrates the cortical and cancellous bone volumes and the related Hounsfield density data. In addition, the main data on the cortical and cancellous bone volumes and related Hounsfield density are summarized in Table 1. Figure 5 shows the data on the cortical vestibular thickness measured on the cross-sectional images of the right canine tooth (3R), the median section of the mandible (M), and the left canine tooth (3L). The main data on the cortical vestibular thickness are summarized in Table 2. Age-related data on cortical/cancellous bone volumes and the related Hounsfield density are shown in Figure 6.
The following is a summary of the main results. The mean value of the cortical volume was 0.71±0.23 mL, ranging from 0.27 mL to 1.96 mL, and that of the cancellous volume was 2.16±0.76 mL, ranging from 0.86 mL to 6.28 mL. The total bone volume was 2.87 mL, which was the sum of the cortical and cancellous bone volumes.
On the right canine tooth (3R) area, the minimal cortical vestibular thickness was 1.55±0.40 mm, ranging from 0.63 mm to 2.87 mm, and the maximal thickness was 3.16±0.70 mm, ranging from 1.54 mm to 5.83 mm. On the median area of the mandible (M), the minimal thickness was 1.53±0.43 mm, ranging from 0.61 mm to 3.25 mm, and the maximal thickness was 3.07±0.73 mm, ranging from 1.01 mm to 4.74 mm. On the left canine tooth (3L) area, the minimal thickness was 1.55±0.41 mm, ranging from 0.82 mm to 3.00 mm, and the maximal thickness was 3.19 mm±0.82 mm, ranging from 1.55 to 5.56 mm. On average of the three areas listed above, the minimal thickness was 1.54±0.41 mm, ranging from 0.61 mm to 3.25 mm, and the maximal thickness was 3.14±0.75 mm, ranged from 1.01 mm to 5.83 mm. Figure 6A shows the volumes of the cortical and cancellous bone with their average lines and the linear trend lines. The volumes of cortical and cancellous bone did not depend on the patients' age.
The Hounsfield densities were 1,087±142 HU, ranging from 608 to 1,349 HU for the cortical bone, and 433±146 HU, ranging from 110 to 869 HU for the cancellous bone. Figure 6B shows the Hounsfield densities of cortical and cancellous bones. The Hounsfield density showed an age-related pattern, that the cancellous bone density increased with age, while the cortical bone density decreased as seen by the linear trend lines.
Rehabilitation of partial or total endentulism patients through the use of dental implants plays a pivotal role nowadays. Insufficient bone volume, reduced height or thickness of the alveolar crest, and loss of normal interarch relationships can make incorrect or impossible implant positioning, either aesthetically or functionally.
Reconstructive surgery can address several possible aims, including the restoration of the correct edentulous ridge morphology, restoration of an edentulous ridge volume sufficient to permit the positioning of the implants, and re-establishment of the correct skeletal inter-mandibular relationships. The present study focused on the evaluation of the inter-foramina area as a donor site. In cases showing a double unilateral mental foramen, the mesial foramen was chosen as the reference margin.
The graft can be harvested as block or particulate bone, and the choice would be made on the basis of a careful pre-operative assessment of the bone defect severity. An important factor in a block bone graft is the microstructure of the harvested bone, such as the cortical and cancellous components (Fig. 7). The microstructure is a crucial factor in determining the degree of revascularization. In cancellous bone, the large marrow spaces between the trabeculae allow a better and faster revascularization, while in cortical bone, the high density of bone lamellae impedes blood vessel development, which will be therefore limited to Volkmann's and Haversian canals.13 Although cancellous bone revascularizes earlier and better on one side, it would be more subjected to resorption on the other side in the presence of compressive forces.14
The CBCT exam provided good resolution images with a voxel dimension of 0.2 mm in our study. CBCT is now commonly used for a variety of purposes in implantology, dentomaxillofacial surgery, image-guided surgical procedures, orthodontics, periodontics, and endodontics.15 In comparison with traditional computed tomography (CT) systems, dental CBCT units offer reduced effective radiation doses, shorter acquisition scan times, easier imaging, and lower costs.16 The main advantages and limits of the CBCT method are described in the review of De Vos et al.17
Many studies have examined the dimensional accuracy of CBCT and the efficiency of the linear and volumetric measurements obtained. Most of them have evaluated the difference between CBCT and multi-slice computed tomography (MSCT), and they found no significant differences or confirmed the correspondence between the linear measurements performed on CBCT and direct measurements on dried anatomical preparations.18,19
However, although recent studies have demonstrated the dimensional reliability of such images,21 all linear and volumetric measurements were performed on the cross sectional images. Bone density values were achieved on radiographic images, too. Maloney et al22 revealed that the linear measurements with digital calipers on the anatomical mandibular sections were accurate and showed no statistically significant differences between the software of a radiographic machine (I-CAT; Imaging Science International, Hatfield, PA, USA) and the implantology design software (SimPlant). These data supported the reliability of the method of measurement used in the experimental protocol of the present study.
Primarily, the limits of the mental region have been defined by Verdugo et al,12 who evaluated the volume that could be safely harvested from the symphysis region during surgery, to preserve the dental element's vitality superiorly, not to damage the inferior alveolar nerve laterally, and not to compromise the structural resistance of the mandible for preservation of the lingual cortical plate inferiorly.
There were few other studies4,12,23-28 that measured the bone volume harvestable from the symphysis region. By the previously reported methodology, the cortical and cancellous bone volumes have been computed. These values should be seen as the maximal volumes harvestable from the symphysis region, in accordance with the safety parameters, and therefore as the largest possible available volume that can be harvested for patient reconstructive requirements. Our study showed that the available bone volume of the donor site did not change depending on the age of the patient (Fig. 6). The harvestable bone would be selected from the basal bone; therefore, it would not be influenced by the crestal bone resorption.
Our study measured the Hounsfield density (HU) using a tool of the SimPlant software. Hounsfield unit is a useful diagnostic tool for quantitative measurement of bone density;29 however, it can be commonly acquired from MSCT. There have been studies proposing the use of HU as a measure of bone density from the CBCT images but they concluded that the HU values obtained in CBCT were different from those in MSCT. In particular, Silva et al30 evaluated the validity of the bone density value in HU from CBCT images compared with MSCT images. They concluded that the use of the HU scale on CBCT images was not a reliable method. Even though the technical improvement of CBCT and the widespread usage of new software enabling "correction" factors would be required for evaluation of bone density as HU, our study evaluated the HU using the SimPlant software, and the HUs were 433±146, and ranging from 110 to 869 for the cancellous bone and 1,087±142, ranging from 608 to 1,349 for the cortical bone. These results were not different from the study of Yavuz et al,27 which reported a mean Hounsfield density of 958.95±98.11 HU for cortical-cancellous bone.
In the light of this new knowledge, the bone classification related to the macroarchitecture should be revised. Davies explained the paradox of the "poor quality" bone.31 According to the study, trabecular bone represented a biologically superior tissue, ideally evolved for rapid (periimplant) bone healing compared with cortical bone, which showed a slowly remodeling healing pattern; therefore, the trabecular bone should definitely not be considered to be "poor quality" bone.
The wide medullary spaces contain mesenchymal progenitor stem cells that can differentiate into osteoblasts, but also vascular structures that are able to provide osteoclast circulating precursors that are required for the remodeling process and the endothelial cell component required for angiogenesis.32 The cancellous bone is, therefore, an important component of the graft.
A further aim of this study was to evaluate the thickness of the cortical vestibular plate of the mental region. Verdugo et al12 reported that the cortical thicknesses were 2.2±0.4 mm, ranging from 1.1 mm to 3.2 mm in the median region and 2.1±0.5 mm, ranging from 1.3 mm to 3.5 mm in the canine region. The values were in line with our results.
The software processing of the CBCT images allows easy assessment of the patient-specific amount of harvestable bone. The procedure exploits the radiographic documentation usually required for reconstruction33 and involves no additional biological costs to the patient.34 The proposed evaluation method might help the surgeon in the selection of the donor site by the comparison between bone availability in the donor site and reconstructive exigency of the recipient site.
Figures and Tables
Fig. 2
The pictures show the procedure of the identification of the mental region. A. The lateral limits are defined as 5 mm in front of the medial margin of each mental foramen. B. The upper limit is defined as 5 mm under the apex of each tooth present and the lower as the upper part of the inferior mandibular cortex.

Fig. 3
A. Cortical bone (yellow) and cancellous bone (green) are defined on the cross-sectional image. B. Three cross-sections are determined at the right canine tooth (3R), median section (M), and left canine tooth (3L). C. The minimal and maximal cortical thickness are measured on the cross-section at the left canine tooth.
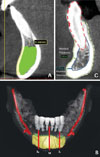
Fig. 5
The cortical plate thickness at the right canine tooth, median section, and left canine tooth.

Fig. 6
The cortical/cancellous bone volume (A) and Hounsfield density (B). (The cases are arranged in order of age and linear trend lines have been drawn.) A. The average lines and linear trend lines coincide and are superimposed, as the volumes of the cortical and cancellous bone did not depend on the patients' age (19-91) in the considered range. B. The Hounsfield densities of the cortical and cancellous bone show an age-related pattern. The cancellous bone density increases with age, while the cortical bone density decreases.

References
1. Chiapasco M, Zaniboni M, Boisco M. Augmentation procedures for the rehabilitation of deficient edentulous ridges with oral implants. Clin Oral Implants Res. 2006; 17:Suppl 2. 136–159.

3. Cawood JI, Howell RA. Reconstructive preprosthetic surgery. I. Anatomical considerations. Int J Oral Maxillofac Surg. 1991; 20:75–82.
4. Yates DM, Brockhoff HC 2nd, Finn R, Phillips C. Comparison of intraoral harvest sites for corticocancellous bone grafts. J Oral Maxillofac Surg. 2013; 71:497–504.

5. Akintoye SO, Lam T, Shi S, Brahim J, Collins MT, Robey PG. Skeletal site-specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals. Bone. 2006; 38:758–768.

7. Silva FM, Cortez AL, Moreira RW, Mazzonetto R. Complications of intraoral donor site for bone grafting prior to implant placement. Implant Dent. 2006; 15:420–426.

8. Raghoebar GM, Louwerse C, Kalk WW, Vissink A. Morbidity of chin bone harvesting. Clin Oral Implants Res. 2001; 12:503–507.

9. Clavero J, Lundgren S. Ramus or chin grafts for maxillary sinus inlay and local onlay augmentation: comparison of donor site morbidity and complications. Clin Implant Dent Relat Res. 2003; 5:154–160.

10. Misch CM. Use of the mandibular ramus as a donor site for onlay bone grafting. J Oral Implantol. 2000; 26:42–49.

11. Pommer B, Tepper G, Gahleitner A, Zechner W, Watzek G. New safety margins for chin bone harvesting based on the course of the mandibular incisive canal in CT. Clin Oral Implants Res. 2008; 19:1312–1316.

12. Verdugo F, Simonian K, Smith McDonald R, Nowzari H. Quantitation of mandibular symphysis volume as a source of bone grafting. Clin Implant Dent Relat Res. 2010; 12:99–104.

15. Kau CH, Bozic M, English J, Lee R, Bussa H, Ellis RK. Conebeam computed tomography of the maxillofacial region - an update. Int J Med Robot. 2009; 5:366–380.
16. Scarfe WC, Farman AG, Sukovic P. Clinical applications of cone-beam computed tomography in dental practice. J Can Dent Assoc. 2006; 72:75–80.
17. De Vos W, Casselman J, Swennen GR. Cone-beam computerized tomography (CBCT) imaging of the oral and maxillofacial region: a systematic review of the literature. Int J Oral Maxillofac Surg. 2009; 38:609–625.

18. Torres MG, Campos PS, Segundo NP, Navarro M, Crusoé-Rebello I. Accuracy of linear measurements in cone beam computed tomography with different voxel sizes. Implant Dent. 2012; 21:150–155.

19. Suomalainen A, Vehmas T, Kortesniemi M, Robinson S, Peltola J. Accuracy of linear measurements using dental cone beam and conventional multislice computed tomography. Dentomaxillofac Radiol. 2008; 37:10–17.

20. Nakano H, Mishima K, Ueda Y, Matsushita A, Suga H, Miyawaki Y, et al. A new method for determining the optimal CT threshold for extracting the upper airway. Dentomaxillofac Radiol. 2013; 42:26397438.

21. Naitoh M, Aimiya H, Hirukawa A, Ariji E. Morphometric analysis of mandibular trabecular bone using cone beam computed tomography: an in vitro study. Int J Oral Maxillofac Implants. 2010; 25:1093–1098.
22. Maloney K, Bastidas J, Freeman K, Olson TR, Kraut RA. Cone beam computed tomography and SimPlant materialize dental Software versus direct measurement of the width and height of the posterior mandible: an anatomic study. J Oral Maxillofac Surg. 2011; 69:1923–1929.

23. Bähr W, Coulon JP. Limits of the mandibular symphysis as a donor site for bone grafts in early secondary cleft palate osteoplasty. Int J Oral Maxillofac Surg. 1996; 25:389–393.

24. Montazem A, Valauri DV, St-Hilaire H, Buchbinder D. The mandibular symphysis as a donor site in maxillofacial bone grafting: a quantitative anatomic study. J Oral Maxillofac Surg. 2000; 58:1368–1371.

25. Güngörmüş M, Yilmaz AB, Erta U, Akgül HM, Yavuz MS, Harorli A. Evaluation of the mandible as an alternative autogenous bone source for oral and maxillofacial reconstruction. J Int Med Res. 2002; 30:260–264.

26. Neiva RF, Gapski R, Wang HL. Morphometric analysis of implant-related anatomy in Caucasian skulls. J Periodontol. 2004; 75:1061–1067.

27. Yavuz MS, Buyukkurt MC, Tozoglu S, Dagsuyu IM, Kantarci M. Evaluation of volumetry and density of mandibular symphysis bone grafts by three-dimensional computed tomography. Dent Traumatol. 2009; 25:475–479.

28. Verdugo F, Simonian K, Raffaelli L, DAddona A. Computeraided design evaluation of harvestable mandibular bone volume: a clinical and tomographic human study. Clin Implant Dent Relat Res. (in press).

29. Shapurian T, Damoulis PD, Reiser GM, Griffin TJ, Rand WM. Quantitative evaluation of bone density using the Hounsfield index. Int J Oral Maxillofac Implants. 2006; 21:290–297.
30. Silva IM, Freitas DQ, Ambrosano GM, Bóscolo FN, Almeida SM. Bone density: comparative evaluation of Hounsfield units in multislice and cone-beam computed tomography. Braz Oral Res. 2012; 26:550–556.

32. Davies JE. Mechanisms of endosseous integration. Int J Prosthodont. 1998; 11:391–401.
33. Pikos MA. Mandibular block autografts for alveolar ridge augmentation. Atlas Oral Maxillofac Surg Clin North Am. 2005; 13:91–107.

34. Tuzi A, Di Bari R, Cicconetti A. 3D imaging reconstruction and impacted third molars: case reports. Ann Stomatol (Roma). 2012; 3:123–131.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download