Abstract
Primary intraosseous squamous cell carcinoma (PIOSCC) is a rare carcinoma, which arises within the jaws without connection to the oral mucosa and presumably develops from a remnant of odontogenic epithelium. We present a case of solid type PIOSCC in a 52-year-old male patient complaining of dull pain on his left lower molar. In this case, early stage PIOSCC mimicking a periapical lesion might lead to a one-year delay in treatment due to the misdiagnosis of osteomyelitis after extraction of the third molar. The clinical, radiological, and histologic features are described. In this case, there was initial radiographic evidence for PIOSCC mimicking a periapical lesion. Incautious radiographic interpretation and treatment procedures had delayed the correct diagnosis and resulted in extensive bony destruction during the patient's disease progression.
Primary intraosseous squamous cell carcinoma (PIOSCC) is a rare carcinoma arising within the jawbone without connection to the oral mucosa, presumably developing from a remnant of odontogenic epithelium. According to the 2005 World Health Organization (WHO) Classification of Tumors, PIOSCC is categorized into 3 types: (1) a solid tumor that invades bone marrow spaces and induces osseous resorption, (2) a squamous cell carcinoma arising from the lining epithelium of an odontogenic cyst, and (3) a squamous cell carcinoma in association with other benign epithelial odontogenic tumors.1
PIOSCC might originate from direct transformation of odontogenic epithelial rests, including the rests found within the periodontal ligament (Malassez's epithelial rest) and within the alveolar bone subsequent to tooth loss, remnants of the dental lamina, and the reduced enamel epithelium surrounding an unerupted or impacted tooth.2
The classification of PIOSCC has been changed whenever there has been a need to reclassify PIOSCC according to the histologic type of odontogenic tumor. It is difficult to confirm a definitive diagnosis for PIOSCC since the diagnostic criteria for PIOSCC remain obscure.
We report a solid type PIOSCC involving the mandible. In this case, late diagnosis occurred due to the radiographic manifestation mimicking periapical disease related to an impacted third molar.
A 52-year-old male patient was referred to the Dental Hospital of Kyung Hee University from a local clinic for the extraction of his left third molar. He had suffered some discomfort during mastication and dull pain on his left lower posterior area beginning about 10 days earlier. On intraoral examination, the overlying mucosa of the left impacted third molar was intact. The shape and texture of the gingiva surrounding the second molar were relatively normal. Neither tenderness nor pus discharge via gingival sulcus was observed during the palpation on the left lower posterior area. Percussion was positive on the second molar, but no mobility was observed. No cervical lymphadenopathy was detected on his left submandibular area. His medical history was noncontributory, revealing controlled hypertension for the past 5 years.
Panoramic and periapical radiographic examination were performed under the clinical diagnosis of pericoronitis. Conventional radiographs showed an ill-defined periapical rarefaction coinciding with the external root resorption related with the follicular space of the impacted third molar. However, there was no any etiologic factor on the left lower second molar such as dental caries or periodontitis (Fig. 1). CBCT examination was performed in order to evaluate the relationship with the mandibular canal and apical radiolucency of the left lower second molar. The CBCT scan described external root resorption on the distal root and extensive bony lysis over the apex of the second molar from the follicular space of the impacted third molar (Fig. 2). The initial radiographic impression was pericoronitis with an advanced lytic bone lesion involving the periapical region of the adjacent root.
He underwent a surgical extraction of his left lower third molar. Despite the continous treatment for 3 months, there was delayed extraction wound healing assuming a surface osteitis.
After one year, he presented to our dental hospital with a complaint of paresthesia in the lower chin area. Intraoral examination revealed that the extraction wound was quite well healed and covered by intact, normal-appearing mucosa on his left mandibular retromolar region. Neither tenderness nor pus discharge was observed during palpation of the extraction wound. No cervical lymph node enlargement was detected in his left submandibular area.
Panoramic radiograph and CBCT scan revealed an ill-defined bony destructive lesion with perforation of the buccal and lingual cortical plates. This lesion extended from the distal root of his left second molar to the ascending ramus and involved the cortical outline of the mandibular canal inferiorly (Fig. 3). The radiographic impression suggested osteomyelitis, and a primary intraosseous malignant tumor was also suspected. Partial sequestrectomy was performed under the tentative diagnosis of osteomyelitis, and a biopsy specimen was obtained for a histopathologic examination. Two specimens were drawn, one was the teeth with attached soft tissue and the other was a brown soft tissue. Microscopic examination revealed infiltrative growth of the neoplastic squamous cells. The tumor cells formed irregular epithelial islands with celluar atypia, nuclear hyperchromatism, pleomorphism, and mitosis (Fig. 4).
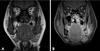
Contrast-enhanced CT scan found a heterogeneously enhanced soft tissue mass in the left retromolar region with extensive bone destruction. MR images revealed adjacent soft tissue involvement, extending laterally into the buccinater and masster muscle, with invasion into the medial pterygoid muscle and masticator space. The tumorous mass showed low signal intensity on the T1 weighted image, while it was detected to have a high signal intensity on the T2 weighted image. Gadolinium-enhanced MRI showed an ill-defined mass with internal heterogeneous enhancement (Fig. 5).
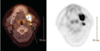
On the findings of CT and MRI, the cervical lymph nodes were found enlarged. As a result, positron emission tomography (PET) and a bone scan were considered to be necessary for evaluating the entity for a primary malignant lesion or long metastatic lesion. According to the results of PET using 18F-fluorodeoxyglucose (FDG), a focal hypermetabolic mass showing a 10.9 standardized uptake value (SUVmax) was detected on the retromolar region of his left mandible (Fig. 6). Furthermore, a hypermetabolic lymph node showing an SUVmax of 5.0 was also observed on his left cervical area. In addition, a Tc99m-MDP bone scintigram revealed an increased uptake of radionuclides in the left mandibular angle area, but no evidence of bone metastasis except the degenerative change of other joints. The patient underwent radiation therapy for three months before surgical intervention. Under general anesthesia, the patient underwent partial mandibulectomy and selective neck dissection. The surgical defect was also reconstructed by surgical plates and a radial forearm flap. The histopathologic examination of the surgical specimen proved that the tumor, measuring about 4 cm×3.5 cm×3 cm, invaded the cortical bone of the mandible and surrounding skeletal muscles. The tumor cells formed an atypical epithelial cell nest with hyperchromatism and pleomorphism, while the overlying oral mucosa had no connection with the tumor. Scetions of a regional lymph node showed no tumoral cells. The histological diagnosis was moderately differentiated squamous cell carcinoma most likely of central origin. No lymphatic metastasis was detected; however, tumor cells were very close to lingual and buccal resection margins. In conclusion, the diagnosis of PIOSCC was confirmed radiographically and histopathologically. Up to the present, there has been no evidence of local recurrence or distant metastasis during the six-month postoperative follow-up period.
PIOSCC is a rare carcinoma that arises by direct transformation of odontogenic epithelial rests in the jawbone, including the epithelial rests found within the periodontal ligament and alveolar bone, remnants of the dental lamina, and the reduced enamel epithelium surrounding an unerupted/impacted tooth.1,2
Pindborg et al3 coined the term primary intraosseous carcinoma (PIOC) in the first edition of the WHO classification for histological typing of odontogenic tumors. Elazy4 subsequently recommended a modification of this WHO classification after reviewing a sample of subjects with PIOC. The classification was further modified in 1984,5 and in 1989, intraosseous mucoepidermoid carcinoma was added to the classification of PIOC.6 In 2005, the WHO used the term PIOSCC and categorized it into 3 types:1 (1) a solid tumor that invades bone marrow spaces and induces osseous resorption, (2) a squamous cell carcinoma arising from the lining epithelium of an odontogenic cyst, and (3) a squamous cell carcinoma in association with other benign epithelial odontogenic tumors.
The diagnostic criteria are obscure, but the followings are suggested as the criteria for PIOSCC:7 (1) It should be squamous cell carcinoma in a histopathologic basis without any other odontogenic cyst nor metastatic tumor cells. (2) It should have intact mucosa. (3) There is no another distant primary tumor at the time of diagnosis and at least 6 month's absence of malignancy during the follow-up period.
According to Lugakingira et al,8 there was a predilection for this lesion to occur in men (male : female ratio, 3 : 1). Most de novo PIOSCC developed in the molar-ramus region of the mandible. Most patients were between 24 and 76 years old, with >80% in the fifth to seventh decades. The most frequent clinical features were swelling, pain/toothache. As the lesion grew, numbness and trimus followed as symptoms due to the mandibular nerve and muscle infiltration.
Radiographic examination is one of the most effective means of detecting PIOSCC. However, PIOSCC usually shows great variation in size, shape, and in the appearance of the border.9,10 In most cases, PIOSCCs show ill-defined bone destructive lesions with a ragged border on plain radiographs. However, in some cases, PIOSCC mimicking periapical lesion and periodontal lesion can lead to misdiagnosis.7,11 In this case, there were initial radiographic features showing atypical periapical rarefaction demanding advanced careful radiographic interpretation due to the absence of related etiological factors.
Vague pain and paraesthesia, which are some of the typical clinical features of PIOSCC, make practitioners misdiagnose that tumor as a simple dental problem.12,13 As a result, this fact may increase the possibility of late diagnosis. To et al14 described the delay in correct diagnosis as ranging from a few weeks to as long as 18 months. In this case, the ill-defined osteolytic lesion was regarded as osteomyelitis or an extraction wound having delayed-healing for several months due to the extraction history and involvement of the impacted third molar.
In the most recent review by Lugakingira et al8 for the prompt diagnosis of PIOSCC, advanced imaging modalities such as PET and CT scan were preferred. PET is very useful for detecting another primary cancer or long metastasis during 6-month follow-up, while CT scanning provides detailed information about the location, size, and shape of the lesion. MR features of PIOSCC showed various signal intensities reflecting their polymorphic features. The solid portions showed various signal intensities, ranging from low to high signal intensity on both T1 and T2 weighted images as well as contrast enhancement. However, the cystic portion showed homogeneous low-to-high signal intensity on T1WI and homogeneous slight high-to-high signal intensity on T2WI with no contrast enhancement, reflecting cystic features of a water-like component. The absence of the cystic portion is good information for diagnosis of PIOSCC before histologic study.
According to Woolgar's review, local recurrence is reported in almost 20% of cases, and regional and distant metastases (predominantly pulmonary, ribs, and lumbar vertebrae) in almost 30%. The reported 2-year survival rate of 30% to 40% is consistent with other stage IV oral cavity lesions. Prognosis is related to the duration from the complaint to diagnosis. Radical resection has been widely accepted as the treatment of PIOSCC. Other treatment methods such as radiotherapy and chemotherapy may be considered in cases where surgical resection is inadequate.15
PIOSCC originating de novo is rare, but if it is a small lesion like the present case, PIOSCC may resemble a periapical lesion or other radiolucent lesion. This should be included in the differential diagnosis of other radiolucent lesions or bone destructive lesions. In this case, the initial radiographic evidence for PIOSCC mimicked a periapical lesion. An incautious radiographic interpretation and treatment procedure had delayed the correct diagnosis and resulted in extensive bony destruction in the progression of the patient's disease.
Figures and Tables
Fig. 1
Cropped panoramic (A) and periapical (B) radiographs show an ill-defined periapical rarefaction coinciding with external root resorption related with the follicular space of the impacted third molar.
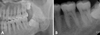
Fig. 2
CBCT images show external root resorption on the distal root and extensive bony lysis over the apex of the second molar from the follicular space of the impacted third molar.
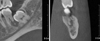
Fig. 3
CBCT images reveal an extensive and ill-defined bony destruction with the perforation of the buccal and lingual cortical plates.

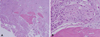
Fig. 4
Photomicrographs of the lesion show moderately differentiated squamous cells and solid tumor tissue with cellular atypia and pleomorphism. (H&E stain, A. ×40, B. ×400)
References
1. Barnes L, Eveson JW, Reichart P, Sidransky D. World Health Organization classification of tumours: Pathology and genetics of head and neck tumours. 2005. Lyon: IARC Press;290–291.
2. Eversole LR. Malignant epithelial odontogenic tumors. Semin Diagn Pathol. 1999. 16:317–324.
3. Pindborg JJ, Kramer IR, Torloni H. International histological classification of tumours, No. 5. Histological typing of odontogenic tumours, jaw cysts, and allied lesions. 1971. Geneva: World Health Organization;35–36.
4. Elzay RP. Primary intraosseous carcinoma of the jaws. Review and update of odontogenic carcinoma. Oral Surg Oral Med Oral Pathol. 1982. 54:299–303.
5. Slootweg PJ, Müller H. Malignant ameloblastoma or ameloblastic carcinoma. Oral Surg Oral Med Oral Pathol. 1984. 57:168–176.

6. Waldron CA, Mustoe TA. Primary inraosseous carcinoma of the mandible with probable origin in an odotogenic cyst. Oral Surg Oral Med Oral Pathol. 1989. 67:716–724.
7. Suei Y, Tanimoto K, Taguchi A, Wada T. Primary intraosseous carcinoma: review of the literature and diagnostic criteria. J Oral Maxillofac Surg. 1994. 52:580–583.

8. Lugakingira M, Pytynia K, Kolokythas A, Miloro M. Primary intraosseous carcinoma of the mandible: case report and review of the literature. J Oral Maxillofac Surg. 2010. 68:2623–2629.

9. Kaffe I, Ardekian L, Peled M, Machtey E, Laufer D. Radiological features of primary intra-osseous carcinoma of the jaws. Analysis of the literature and report of a new case. Dentomaxillofac Radiol. 1998. 27:209–214.

10. Thomas G, Pandey M, Mathew A, Abraham EK, Francis A, Somanathan T, et al. Primary intraosseous carcinoma of the jaw: pooled analysis of world literature and report of two new cases. Int J Oral Maxillofac Surg. 2001. 30:349–355.

11. Hwang EH, Choi YS, Lee SR. Primary intraosseous carcinoma of the mandible. Korean J Oral Maxillofac Radiol. 2005. 35:235–239.
12. Thomas G, Sreelatha KT, Balan A, Ambika K. Primary intaosseous carcinoma of the mandible - a case report and review of the literature. Eur J Surg Oncol. 2000. 26:82–86.
13. Dimitrakopoulos I, Psomaderis K, Asimaki A, Papaemanouel S, Karakasis D. Primary de novo intraosseous carcinoma: report of two cases. J Oral Maxillofac Surg. 2005. 63:1227–1230.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download