Abstract
Central odontogenic fibroma is a rare odontogenic neoplasm that originates from odontogenic ectomesenchyme. Here, a case of central odontogenic fibroma in a 17-year-old male is reported. Since the present case showed a multilocular radiolucency with partially ill-defined border between the right mandibular condyle and the distal root of the right mandibular third molar, differential diagnosis involved a wide range of pathosis from benign lesions like ameoloblastic fibroma and odontogenic myxoma to more aggressive lesions such as desmoplastic fibroma, juvenile aggressive fibromatosis, or fibrosarcoma.
Odontogenic fibroma (ODF) originates from odontogenic ectomesenchyme. ODF can be further divided into central (intraosseous) odontogenic fibroma (CODF) and peripheral (extraosseous) odontogenic fibroma (PODF) according to the anatomical sites involved.1
The CODF is a rare odontogenic neoplasm. Regezi et al2 were unable to identify any ODFs among 706 odontogenic tumors retrieved from 54,534 oral specimens. Handers et al3 identified only 19 CODFs from over 80,000 accessioned oral specimens. In 40,000 consecutive oral biopsies from a Canadian population, there were only 25 CODFs (0.06%) and 36 PODFs (0.09%).4 Buchner et al5 have shown 23 PODFs (0.02%) and 16 CODFs (0.02%) among 91,178 oral lesions. Recently Lin et al6 reported 15 cases of ODFs including 3 cases of central type. Although PODF is relatively rare, it is the most common peripheral odontogenic tumor (51%, 23/45).7 Moreover, PODF is more common than its central counterpart by a ratio of 1.4 : 1.7
Here, a case of CODF is presented because due to its extensive bone destruction with partially ill-defined margin, differential diagnosis involved a wide range of pathosis.
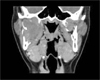
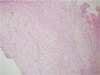
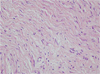
A 17-year-old male was referred to our hospital for evaluation of a painless swelling on the right cheek, which had been identified 3 or 4 months earlier. His medical and surgical history was noncontributory. The extraoral examination showed swelling on the right cheek causing some facial asymmetry (Fig. 1). The intraoral examination revealed a slight swelling on the right mandiblular ramus region, extending to the right retromolar area (Fig. 2). The mass was firm with normal color and no fluctuation was evident on palpation. There was no associated cervical lymphadenopathy. The panoramic radiographic examination showed the presence of a multilocular radiolucency with partially ill-defined border between the right mandibular condyle and the distal root of the right mandibular third molar. Since the radiolucency was not associated with the crown of the impacted third molar and seemed to involve the mandibular foramen, the first radiologic impression involved neural origin tumor along with more aggressive lesions such as desmoplastic fibroma, juvenile aggressive fibromatosis, or fibrosarcoma (Fig. 3). CT examination showed an expansile solid ovoid slightly enhancing mass involving the right mandibular condyle and ramus, measuring around 3.5×3.2 cm giving the impression of an ameloblastoma (Figs. 4, 5, 6). Microscopic examination revealed a non-encapsulated tumor consisting of bland fibroblast cells with wavy cytoplasm. A few strands and nests of odontogenic epithelium were observed (Figs. 7 and 8). Based on the clinical, radiographic, and histopathologic findings, the definitive diagnosis was CODF. The patient showed no clinical signs of recurrence 2 years after surgical excision.
According to Covani et al,8 the CODF was believed to originate from mesenchymal odontogenic tissue such as dental papilla, periodontal ligament, or dental follicle. Considering the histogenesis of the lesion in the WHO classification,9 it has been suggested that the epithelium-poor type of CODF is derived from the dental follicle, whereas the epithelium-rich type arises from the periodontal ligament.
The peak incidence of CODFs have been observed in the second decade of life and shows a female predilection.10 This lesion was originally thought to occur almost exclusively in the mandible, and was mostly found in the posterior mandible.11 Clinically, CODF manifests as a painless swelling and has a slow growth that results in cortical expansion.10,11 Clinical symptoms such as pain and paresthesia are uncommon.12
Radiologically, most cases of CODFs are radiolucent, unilocular, and with well-defined borders similar to other radiolucent lesions such as traumatic bone cyst, ameloblastoma, odontogenic cysts, and central giant cell granulomas.13 Araki et al14 reported that the diagnosis of CODF by radiographic findings was extremely difficult, especially when the lesion was associated with a crown of the unerupted tooth that might resemble a dentigerous cyst. Since the present case showed a multilocular radiolucent lesion involving ramus and even condylar process with partially ill-defined borders, the differential diagnosis involved a wide range of pathosis from benign lesions such as ameoloblastic fibroma, odontogenic myxoma to more aggressive lesions such as desmoplastic fibroma, juvenile aggressive fibromatosis or fibrosarcoma. Most of the CODF cases reported3,6,8,12 were small and associated with roots of teeth. The CODF case reported by Daniels10 was somewhat bigger in size, however it resembled a dentigerous cyst. Handers et al3 reported that there were only two mandibular lesions among the 19 CODF cases, and one of the mandibular lesions with a large multilocular radiolucency involving most of the ramus was similar to this case except for the fact that the margin was scalloped and showed well-defined buccal cortical expansion suggesting benign nature of the lesion. Kaffe and Buchner13 stated that the majority of CODFs were unilocular radiolucent lesions with well-defined border, however they might also appear as multilocular lesions and might exhibit a mixed radiolucent-radiopaque appearance with poorly defined or diffused borders in rare cases. The great variability in radiologic appearance of the CODFs means that it should be considered in the differential diagnosis of all radiolucencies found in the jaws.
Figures and Tables
Fig. 2
Intraoral photograph shows normal-colored mucosal right cheek swelling extending to the retromolar area.
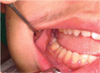
Fig. 3
Cropped panoramic image shows a multilocular radiolucency with partially ill-defined border between the right mandibular condyle and the distal root of the right mandibular third molar.
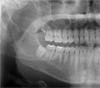
Fig. 4
Axial CT image shows an expansile solid ovoid mass involving the right mandibular condyle and ramus measuring around 3.5×3.2 cm.

Fig. 6
Coronal CT image shows massive bone resorption of the right mandibular condylar process and ramus.
References
1. Neville BW, Damm DD, Allen CM, Bouquot JE. Oral and maxillofacial pathology. 2002. 2nd ed. Philadelphia: WB Saunders;633–635.
2. Regezi JA, Kerr DA, Courtney RM. Odontogenic tumors; analysis of 706 cases. J Oral Surg. 1978. 36:771–778.
3. Handlers JP, Abrams AM, Melrose RJ, Danforth R. Central odontogenic fibroma: clinicopathologic features of 19 cases and review of the literature. J Oral Maxillofac Surg. 1991. 49:46–54.

4. Daley TD, Wysocki GP, Pringle GA. Relative incidence of odontogenic tumors and oral and jaw cysts in a Canadian population. Oral Surg Oral Med Oral Pathol. 1994. 77:276–280.

5. Buchner A, Merrell PW, Carpenter WM. Relative frequency of central odontogenic tumors: a study of 1,088 cases from Northern California and comparison to studies from other parts of the world. J Oral Maxillofac Surg. 2006. 64:1343–1352.

6. Lin HP, Chen HM, Vu CH, Yang H, Kuo RC, Kuo YS, et al. Odontogenic fibroma: a clinicopathological study of 15 cases. J Formos Med Assoc. 2011. 110:27–35.

7. Buchner A, Merrell PW, Carpenter WM. Relative frequency of peripheral odontogenic tumors: a study of 45 new cases and comparison with studies from the literature. J Oral Pathol Med. 2006. 35:385–391.

8. Covani U, Crespi R, Perrini N, Barone A. Central odontogenic fibroma: a case report. Med Oral Patol Oral Cir Bucal. 2005. 10:Suppl 2. E154–E157.
9. Philipsen HP, Reichart PA, Sciubba JJ, van der Waal I. Barnes L, Eveson JW, Reichart PA, Sidransky D, editors. Odontogenic fibroma. World Health Organization classification of tumours. Pathology and genetics of tumours of head and neck tumours. 2005. Lyon: IARC;317.
10. Daniels JS. Central odontogenic fibroma of mandible: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004. 98:295–300.

12. Daskala I, Kalyvas D, Kolokoudias M, Vlachodimitropoulos D, Alexandridis C. Central odontogenic fibroma of the mandible: a case report. J Oral Sci. 2009. 51:457–461.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download