Abstract
Purpose
The purpose of this study was to obtain the description of the mandibular bone quality of male and female patients between 40-60 years old and their differences based on mandibular cortical bone thickness measured using Mental Index (MI).
Materials and Methods
Forty digital panoramic radiographs, which consisted of twenty male and twenty female patients, 40-60 years old, were observed. Mandibular cortical bone thickness was measured using MI on both sides of the mandible. The average MI score of two groups were then assessed using t-sample independent test.
Osteoporosis is one of the degenerative diseases that can cause health problem in Indonesia recently as a result from increase in life expectancy and life style alteration. Two of five Indonesian women have risk from osteoporosis.1 Diagnosis of osteoporosis is essential to detect fracture risk, especially in high risk group. However, in Indonesia as a developing country, the expenses of the examination still become a major problem. Panoramic radiograph used widely in dentistry is available for early detection of osteoporosis with lower expense. One of the techniques to detect osteoporosis is cortical thickness measurement.
Radiography is utilized as a diagnostic tool for evaluation of teeth and jaw anomalies in dental practice.2,3 Panoramic radiography is one of the most comon radiographies because of its capability to obtain comprehensive image of the maxillofacial structure.4,5 Although dentists mainly focus on teeth and jaw anomalies, it is considered as an obligation for medical practitioners to recognize panoramic images which shows the overall systemic health condition of the patient.6 One of the systemic diseases that reveal themselves as a specific image on panoramic radiograph is osteoporosis. The characteristics of this disease are cortical thinning and more radiolucent trabecular areas.7 Quantity and quality of the jaw bone have important roles in the success of dental care.8
Osteoporosis is diagnosed by examining the BMD score. Unfortunately, BMD tests are very expensive, especially in developing countries such as Indonesia, thus its function as an early detection tool for fracture risks is seldom used. Researches suggested that panoramic radiograph could be a useful identification tool in female with low BMD scores.2 By examining panoramic radiographs, the quality and quantity of bone can be determined.
The purpose of this study was to obtain the description on the mandibular bone quality of male and female between 40-60 years and their differences based on mandibular cortical bone thickness measured using Mental Index (MI) on digital panoramic radiograph.
A total of forty digital panoramic radiographs during the period between January in 2007 and April in 2009 were taken from the files of Dental Hospital of Padjadjaran University, Bandung, Indonesia, to be observed in this study. All of the digital panoramic radiographs were acquired using the same digital panoramic machine (Picasso Trio, Vatech, Seoul, Korea). The radiographs were determined from criteria of clearly visible mental foramina, smooth and continuous mandibular inferior cortical, and without presence of ghost images or double images.
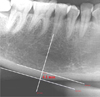
The mandibular cortical thickness was measured bilaterally on the radiographs at the site of the mental foramen as in the previous study of Taguchi et al.16 Briefly, a line parallel to the long axis of the mandible and tangential to the inferior border of the mandible was drawn. A line perpendicular to this tangent line intersecting the inferior border of the mental foramen was constructed, along which the mandibular cortical width was measured using calipers. The mean cortical thickness on both sides of the mandible was defined as Mental Index (MI) in this study.
Mean MI value was recorded for each radiograph and grouped into two groups, male and female, after performing tests of homogeneity of variance. The mean value, standard deviation, and variance were calculated for each group and examined using t-test independent. Age and gender were identified as independent variables and MI was set as the dependent variable (Fig. 1).
Among 40 panoramic radiographs, 20 (50%) were male and 20 (50%) were female. One (5%) radiograph of a male patient had MI score below 3 mm while the rest 19 (95%) radiographs had MI score above 3 mm. Six (30%) radiographs of female patients had MI score below 3 mm while the rest 14 radiographs had MI score above 3 mm. Table 1 shows the distribution of patients according to gender and MI value.
Table 2. shows the examination result using t-test independent of MI value of male and female group.
The mean MI value was 3.928 mm for male group and 3.155 mm for female group. There was a significant difference of MI value between male and female group (p<0.05).
Genetic factors, particularly gender, affected bone mass. Sexual hormone between male and female are different. However, both of them affected bone growth. Testosterone plays a role in male, while estrogen in female to encourage bone growth.9 Bone mass increases constantly and reach the peak bone mass at the age of 40 in male and around age of 30-35 in female.7
Different from male, the aging process in female comes earlier. The reproductive age in female is over at the age of 45-50 when the menstruation cycle ends and sexual hormone rapidly decreases, well known as menopause.10 Estrogen deficiency plays a very important role on menopause as a cause of bone mass decrease.7 Estrogen prevents osteoporosis by inhibiting the stimulation effect on specific cytokines in the osteoclast.11 Decreased level of estrogen will increase the sensitivity of osteoclast to parathyroid hormone. Moreover, estrogen deficiency affects the active vitamin D synthesis in renal tubules and lead to reduction of calcium absorption.12
The diagnosis of osteoporosis can be performed by evaluation of the changes in bone mineral density.13 BMD testing for all post-menopausal women can reduce the incidence of the fracture and complications from osteoporosis, however it is still difficult to perform in clinical practice due to cost issues, limited facilities, and also limited experts.2
In the previous studies, it has been shown that the decreased bone mineral density affected the morphometric, densitometric, and architectural properties of mandibular bone in the osteoporotic patients.14 There were studies that cortical bone thinning occurred especially in menopausal women on panoramic radiograph.15 Cortical thinning on mandible happens as a result from Haversian canal widening.16 One technique that can be used for evaluation of bone quality is the measurement of mandibular cortical thickness which is Mental Index (MI).
The cortical thicknesses of male and female at the age of 40-60 were significantly different (p<0.05) in this study. Female group showed thinner cortical thickness compared with male group. The previous study by Dutra et al on different gender, dentition status, and age of patients revealed that MI was significantly smaller in older females, whereas it was greater for older males (p<0.01). Their result was in accord with our result.17
The threshold of MI score to refer a patient for BMD testing was 3.0 mm, which was based on a previous study in British women population. Another study on Japanese population suggests a different threshold value of 2.8 mm or less to refer a patient for BMD testing.16
This study has some limitations for not including factors that might affect jaw bone quality, such as drugs, regular exercise, smoking, alcoholism, systemic disease, and others. Therefore, we could not compare the cortical thicknesses of normal and osteoporotic patients.
Figures and Tables
References
1. Research and Development Center of Nutrient. Report on the survey of risk. 2006. Jakarta: Ministry of Health Republic of Indonesia.
2. Taguchi A, Suei Y, Sanada M, Ohtsuka M, Nakamoto T, Sumida H, et al. Validation of dental panoramic radiography measures for identifying postmenopausal women with spinal osteoporosis. AJR Am J Roentgenol. 2004. 183:1755–1760.

3. Güngör K, Akarslan Z, Akdevelioglu M, Erten H, Semiz M. The precision of the panoramic mandibular index. Dentomaxillofac Radiol. 2006. 35:442–446.

4. Schulze R, Krummenauer F, Schalldach F, d'Hoedt B. Precision and accuracy of measurements in digital panoramic radiography. Dentomaxillofac Radiol. 2000. 29:52–56.

5. Whaites E. Essentials of dental radiography and radiology. 2002. 3rd ed. Edinburgh: Churchill Livingstone;161–176.
6. Watanabe PC, Farman A, Watanabe MG, Issa JP. Radiographic signals detection of systemic disease. Int J Morphol. 2008. 26:915–926.
7. Devlin H, Horner K. Mandibular radiomorphometric indices in the diagnosis of reduced skeletal bone mineral density. Osteoporos Int. 2002. 13:373–378.

8. Hildebolt CF, Fletcher G, Yokoyama-Crothers N, Conover GL, Vannier MW. A comparison of the response of storage phosphor and film radiography to small variations in X-ray exposure. Dentomaxillofac Radiol. 1997. 26:147–151.

9. Sherwood L. Human physiology: from cells to systems. 2010. 7th ed. Belmont: Brooks/Cole.
10. Bozic M, Ihan Hren N. Osteoporosis and mandibles. Dentomaxillofac Radiol. 2006. 35:178–184.
11. Ganong WF. Review of medical physiology. 2005. 22nd ed. New York: McGraw-Hill Medical.
12. Kumar V, Cotran R, Robins SL. Robbbins basic pathology. 2003. 7th ed. Philadelphia: Saunders.
13. Miliuniene E, Alekna V, Peciuliene V, Tamulaitiene M, Maneliene R. Relationship between mandibular cortical bone height and bone mineral density of lumbar spine. Stomatologija. 2008. 10:72–75.
14. Watanabe PC, Issa JP, Oliveira TM, Monteiro SA, Iyomasa MM, Regalo SC, et al. Morphodigital study of the mandibular trabecular bone in panoramic radiographs. Int J Morphol. 2007. 25:875–880.

15. Gulsahi A, Yüzügüllü B, Ĭmirzalioğlu P, Genç Y. Assessment of panoramic radiomorphometric indices in Turkish patients of different age groups, gender and dental status. Dentomaxillofac Radiol. 2008. 37:288–292.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download