1. Khera M. Controversies in testosterone supplementation therapy. Asian J Androl. 2015; 17:175–176. PMID:
25652639.

2. Zmuda JM, Cauley JA, Kriska A, Glynn NW, Gutai JP, Kuller LH. Longitudinal relation between endogenous testosterone and cardiovascular disease risk factors in middle-aged men. A 13-year follow-up of former Multiple Risk Factor Intervention Trial participants. Am J Epidemiol. 1997; 146:609–617. PMID:
9345114.

3. Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Baltimore Longitudinal Study of Aging. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001; 86:724–731. PMID:
11158037.
4. Bhattacharya RK, Khera M, Blick G, Kushner H, Miner MM. Testosterone replacement therapy among elderly males: the Testim Registry in the US (TRiUS). Clin Interv Aging. 2012; 7:321–330. PMID:
22956867.

5. Malik RD, Lapin B, Wang CE, Lakeman JC, Helfand BT. Are we testing appropriately for low testosterone?: characterization of tested men and compliance with current guidelines. J Sex Med. 2015; 12:66–75. PMID:
25382540.

6. Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010; 95:2536–2559. PMID:
20525905.

7. Wang C, Nieschlag E, Swerdloff R, Behre HM, Hellstrom WJ, Gooren LJ, et al. Investigation, treatment and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA and ASA recommendations. Eur J Endocrinol. 2008; 159:507–514. PMID:
18955511.

8. Nieschlag E, Swerdloff R, Behre HM, Gooren LJ, Kaufman JM, Legros JJ, et al. Investigation, treatment and monitoring of late-onset hypogonadism in males. ISA, ISSAM, and EAU recommendations. Eur Urol. 2005; 48:1–4. PMID:
15951102.

9. US Food and Drug Administration. FDA cautions about using testosterone products for low testosterone due to aging; requires labeling change to inform of possible increased risk of heart attack and stroke with use [Internet]. Silver Spring (MD): US Food Drug Administration;c2015. cited 2017 Sep 6. Available from:
https://www.fda.gov/Drugs/DrugSafety/ucm436259.
10. Corona G, Sforza A, Maggi M. Testosterone replacement therapy: long-term safety and efficacy. World J Mens Health. 2017; 35:65–76. PMID:
28497912.

11. Corona G, Maggi M. Perspective: regulatory agencies' changes to testosterone product labeling. J Sex Med. 2015; 12:1690–1693. PMID:
26289540.

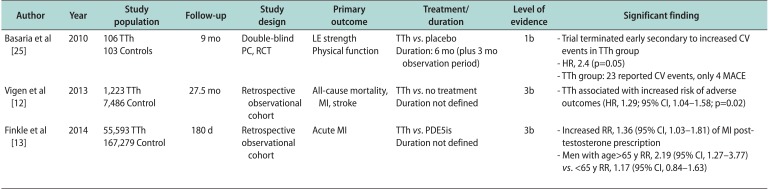
12. Vigen R, O'Donnell CI, Barón AE, Grunwald GK, Maddox TM, Bradley SM, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013; 310:1829–1836. PMID:
24193080.

13. Finkle WD, Greenland S, Ridgeway GK, Adams JL, Frasco MA, Cook MB, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014; 9:e85805. PMID:
24489673.

15. Morgentaler A, Miner MM, Caliber M, Guay AT, Khera M, Traish AM. Testosterone therapy and cardiovascular risk: advances and controversies. Mayo Clin Proc. 2015; 90:224–251. PMID:
25636998.

16. Nieschlag E, Nieschlag S. Testosterone deficiency: a historical perspective. Asian J Androl. 2014; 16:161–168. PMID:
24435052.

17. Beaser SB, Massell TB. Therapeutic evaluation of testosterone in peripheral vascular disease. N Engl J Med. 1942; 227:43–44.

18. Edwards EA, Hamilton JB, Duntley SQ. Testosterone propionate as a therapeutic agent in patients with organic disease of the peripheral vessels: preliminary report. N Engl J Med. 1939; 220:865.
19. Lesser MA. Testosterone propionate therapy in one hundred cases of angina pectoris. J Clin Endocrinol Metab. 1946; 6:549–557. PMID:
20996894.

20. Levine SA, Likoff WB. The therapeutic value of testosterone propionate in angina pectoris. N Engl J Med. 1943; 229:770–772.

21. Moskovic DJ, Araujo AB, Lipshultz LI, Khera M. The 20-year public health impact and direct cost of testosterone deficiency in U.S. men. J Sex Med. 2013; 10:562–569. PMID:
23035926.

22. Khaw KT, Dowsett M, Folkerd E, Bingham S, Wareham N, Luben R, et al. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) Prospective Population Study. Circulation. 2007; 116:2694–2701. PMID:
18040028.
23. Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008; 93:68–75. PMID:
17911176.

24. Haring R, Völzke H, Steveling A, Krebs A, Felix SB, Schöfl C, et al. Low serum testosterone levels are associated with increased risk of mortality in a population-based cohort of men aged 20–79. Eur Heart J. 2010; 31:1494–1501. PMID:
20164245.

25. Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010; 363:109–122. PMID:
20592293.

26. Srinivas-Shankar U, Roberts SA, Connolly MJ, O'Connell MD, Adams JE, Oldham JA, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010; 95:639–650. PMID:
20061435.

27. Incorrect language. JAMA. 2014; 311:306. PMID:
24430330.
28. Incorrect number of excluded patients reported in the text and figure. JAMA. 2014; 311:967.
29. Morgentaler A, Lunenfeld B. Testosterone and cardiovascular risk: world's experts take unprecedented action to correct misinformation. Aging Male. 2014; 17:63–65. PMID:
24797617.

30. Fisher ES, Whaley FS, Krushat WM, Malenka DJ, Fleming C, Baron JA, et al. The accuracy of Medicare's hospital claims data: progress has been made, but problems remain. Am J Public Health. 1992; 82:243–248. PMID:
1739155.

32. Quan A, Chakravarty S, Chen JK, Chen JC, Loleh S, Saini N, et al. Androgens augment proximal tubule transport. Am J Physiol Renal Physiol. 2004; 287:F452–F459. PMID:
15100096.

33. Quigley R. Androgens stimulate proximal tubule transport. Gend Med. 2008; 5(Suppl A):S114–S120. PMID:
18395677.

34. Ajayi AA, Mathur R, Halushka PV. Testosterone increases human platelet thromboxane A2 receptor density and aggregation responses. Circulation. 1995; 91:2742–2747. PMID:
7758179.
35. Ohlander SJ, Varghese B, Pastuszak AW. Erythrocytosis following testosterone therapy. Sex Med Rev. 2017; S2050-0521(17)30041-0. DOI:
10.1016/j.sxmr.2017.04.001.

36. Jeong SK, Rosenson RS. Shear rate specific blood viscosity and shear stress of carotid artery duplex ultrasonography in patients with lacunar infarction. BMC Neurol. 2013; 13:36. PMID:
23597083.

37. Gijsen F, van der Giessen A, van der Steen A, Wentzel J. Shear stress and advanced atherosclerosis in human coronary arteries. J Biomech. 2013; 46:240–247. PMID:
23261245.

38. Gagliano-Jucá T, Basaria S. Trials of testosterone replacement reporting cardiovascular adverse events. Asian J Androl. 2017; DOI:
10.4103/aja.aja_28_17. [Epub].

39. Snyder PJ, Bhasin S, Cunningham GR, Matsumoto AM, Stephens-Shields AJ, Cauley JA, et al. Effects of testosterone treatment in older men. N Engl J Med. 2016; 374:611–624. PMID:
26886521.

40. Budoff MJ, Ellenberg SS, Lewis CE, Mohler ER 3rd, Wenger NK, Bhasin S, et al. Testosterone treatment and coronary artery plaque volume in older men with low testosterone. JAMA. 2017; 317:708–716. PMID:
28241355.

41. Wallis CJ, Lo K, Lee Y, Krakowsky Y, Garbens A, Satkunasivam R, et al. Survival and cardiovascular events in men treated with testosterone replacement therapy: an intention-to-treat observational cohort study. Lancet Diabetes Endocrinol. 2016; 4:498–506. PMID:
27165609.

42. Anderson JL, May HT, Lappé DL, Bair T, Le V, Carlquist JF, et al. Impact of testosterone replacement therapy on myocardial infarction, stroke, and death in men with low testosterone concentrations in an integrated health care system. Am J Cardiol. 2016; 117:794–799. PMID:
26772440.

43. Maggi M, Wu FC, Jones TH, Jackson G, Behre HM, Hackett G, et al. Testosterone treatment is not associated with increased risk of adverse cardiovascular events: results from the Registry of Hypogonadism in Men (RHYME). Int J Clin Pract. 2016; 70:843–852. PMID:
27774779.

44. Traish AM, Haider A, Haider KS, Doros G, Saad F. Long-term testosterone therapy improves cardiometabolic function and reduces risk of cardiovascular disease in men with hypogonadism: a real-life observational registry study setting comparing treated and untreated (control) groups. J Cardiovasc Pharmacol Ther. 2017; 22:414–433. PMID:
28421834.
45. Sharma R, Oni OA, Gupta K, Sharma M, Sharma R, Singh V, et al. Normalization of testosterone levels after testosterone replacement therapy is associated with decreased incidence of atrial fibrillation. J Am Heart Assoc. 2017; 6:e004880. DOI:
10.1161/JAHA.116.004880. PMID:
28487389.

46. Lai J, Zhou D, Xia S, Shang Y, Want L, Zheng L, et al. Reduced testosterone levels in males with lone atrial fibrillation. Clin Cardiol. 2009; 32:43–46. PMID:
19143004.

47. Magnani JW, Moser CB, Murabito JM, Sullivan LM, Wang N, Ellinor PT, et al. Association of sex hormones, aging, and atrial fibrillation in men: the Framingham Heart Study. Circ Arrhythm Electrophysiol. 2014; 7:307–312. PMID:
24610804.
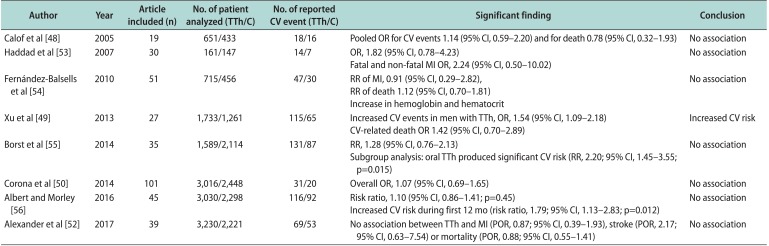
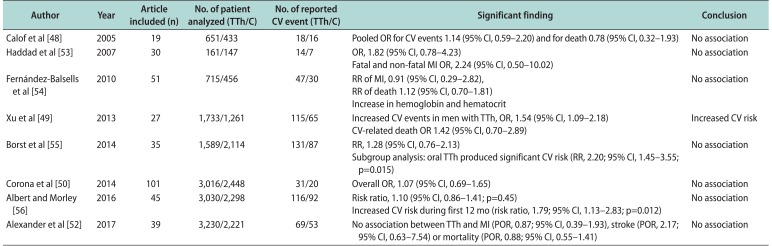
48. Calof OM, Singh AB, Lee ML, Kenny AM, Urban RJ, Tenover JL, et al. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci. 2005; 60:1451–1457. PMID:
16339333.

49. Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med. 2013; 11:108. PMID:
23597181.

50. Corona G, Maseroli E, Rastrelli G, Isidori AM, Sforza A, Mannucci E, et al. Cardiovascular risk associated with testosterone-boosting medications: a systematic review and meta-analysis. Expert Opin Drug Saf. 2014; 13:1327–1351. PMID:
25139126.

51. Onasanya O, Iyer G, Lucas E, Lin D, Singh S, Alexander GC. Association between exogenous testosterone and cardiovascular events: an overview of systematic reviews. Lancet Diabetes Endocrinol. 2016; 4:943–956. PMID:
27669646.

52. Alexander GC, Iyer G, Lucas E, Lin D, Singh S. Cardiovascular risks of exogenous testosterone use among men: a systematic review and meta-analysis. Am J Med. 2017; 130:293–305. PMID:
27751897.

53. Haddad RM, Kennedy CC, Caples SM, Tracz MJ, Boloña ER, Sideras K, et al. Testosterone and cardiovascular risk in men: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007; 82:29–39. PMID:
17285783.

54. Fernández-Balsells MM, Murad MH, Lane M, Lampropulos JF, Albuquerque F, Mullan RJ, et al. Clinical review 1: adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2010; 95:2560–2575. PMID:
20525906.
55. Borst SE, Shuster JJ, Zou B, Ye F, Jia H, Wokhlu A, et al. Cardiovascular risks and elevation of serum DHT vary by route of testosterone administration: a systematic review and metaanalysis. BMC Med. 2014; 12:211. PMID:
25428524.

56. Albert SG, Morley JE. Testosterone therapy, association with age, initiation and mode of therapy with cardiovascular events: a systematic review. Clin Endocrinol (Oxf). 2016; 85:436–443. PMID:
27124404.

57. The Copenhagen. Testosterone treatment of men with alcoholic cirrhosis: a double-blind study. Hepatology. 1986; 6:807–813. PMID:
2875927.

58. Sheffield-Moore M, Dillon EL, Casperson SL, Gilkison CR, Paddon-Jones D, Durham WJ, et al. A randomized pilot study of monthly cycled testosterone replacement or continuous testosterone replacement versus placebo in older men. J Clin Endocrinol Metab. 2011; 96:E1831–E1837. PMID:
21865352.
59. Ismaeel N, Wang R. Testosterone replacement-freedom from symptoms or hormonal shackles? Sex Med Rev. 2017; 5:81–86. PMID:
27444060.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download