INTRODUCTION
The prevalence of obesity has increased over the last several decades in the USA and other countries because of lifestyle changes within the population [
1]. Obesity is a crucial health problem because it is a well-known risk factor for common lifestyle diseases, such as diabetes mellitus, hypertension, and ischemic heart disease [
2]. Furthermore, there is accumulating evidence that obesity is significantly associated with an increased risk of cancer development, recurrence, and cancer-related death for various types of cancer, including prostate cancer (PCa) [
345]. Considering that obesity commonly affects men, the relationship between obesity and PCa is a clinically important issue [
6]. However, previous epidemiologic studies have yielded conflicting results, and the underlying molecular mechanisms of the impact of obesity on PCa biology has yet to be clarified.
At the molecular level, patients with obesity have excessive amount of adipose tissue, and this tissue can produce various proteins, such as leptin, adiponectin, tumor necrosis factor (TNF)-α, and interleukin (IL)-6, which are classified as adipokines [
7]. Factors secreted by adipocytes play an important role in communication with distant tissues and organs, and ultimately regulate metabolic homeostasis in the body [
8]. In patients with PCa, circulating adipokines can influence the several carcinogenic mechanisms of prostate cells [
9]. Although many studies have explored the association between the profiles of adipokines and characteristics of cancer in patients with PCa with or without obesity, these studies have primarily analyzed Western populations.
Considering the different features of PCa between Western and Asian populations, we investigated the profiles of several circulating adipokines according to body mass index (BMI) in Korean patients with localized PCa treated with radical prostatectomy (RP) by prospectively collecting blood samples before surgery. Moreover, we sought to identify the predictors of advanced pathologic stage of PCa in these patients. We hypothesized that the baseline profiles of adipokines and significant predictors would be different between the non-obese and obese groups.
Go to :

MATERIALS AND METHODS
1. Ethics statement
After the Institutional Review Board of Seoul National University Bundang Hospital approved this study (No. B-1506-302-302), we prospectively enrolled 65 patients with clinically localized PCa who underwent RP between July 2015 and March 2016. We obtained written informed consent from all patients, and personal identifiers were removed from the data with anonymized processing. All study protocols, including human resources, were approved by our hospital and were carried out in accordance with the Declaration of Helsinki guidelines.
2. Study sample
Eligible patients were men aged between 40 and 80 years with a diagnosis of adenocarcinoma of the prostate gland, no evidence of radiological metastatic disease in either the lymph nodes or distant organs, and clinically localized disease treated with RP. Three patients were excluded because their samples failed to pass the quality control step before analysis. Of the 65 patients who were initially enrolled, we ultimately analyzed 62 patients with PCa who underwent RP.
3. Study design
The enrolled patients were classified into 2 groups according to their BMI: non-obese (<25 kg/m
2) and obese (≥25 kg/m
2), based on the Clinical Practice Guidelines for Overweight and Obesity in Korea [
10]. We collected data using the following various clinicopathological variables: age at surgery, BMI, diabetes mellitus, preoperative serum prostate-specific antigen (PSA) levels, preoperative serum adipokine and total cholesterol levels, digital rectal examination findings, total prostate volume assessed by transrectal ultrasonography, clinical T stage, biopsy Gleason scores (GS), pathological T stage and GS, extraprostatic extension (EPE), seminal vesicle invasion, and positive surgical margin.
For the analysis of preoperative serum adipokine levels, we collected 10 mL of blood from enrolled patients at our outpatient clinic before surgery. Blood samples were stored at 4℃ and transferred to the Clinical Laboratory Services department of Green Cross LabCell (Yongin, Korea) for the quantitative analysis of circulating adipokine levels. The following 7 key adipokines were measured using their respective detection kits: IL-6 (pg/mL) by the Quantikine HS Human IL-6 Immunoassay (R&D Systems, Inc., Minneapolis, MN, USA), insulin-like growth factor 1 (IGF-1; ng/mL) by LIAISON IGF-1 (Diasorin, Saluggia, Italy), chemerin (pg/mL) by the Human Chemerin Immunoassay (R&D Systems, Inc.), C-X-C motif chemokine 10 (CXCL10; pg/mL) by the hIP-10 Qkit (R&D Systems, Inc.), adiponectin (µg/mL) by the Human Adiponectin ELISA kit (BioVendor, Karasek, Czech Republic), leptin (ng/mL) by the Human Leptin RIA kit (LINCO Research, Inc., St. Charles, MO, USA), and resistin (ng/mL) by the Human Resistin ELISA Kit (AdipoGen, San Diego, CA, USA).
4. Statistical analysis
We conducted a descriptive analysis of the clinicopathological characteristics of the enrolled patients. The results are presented as the proportion of relevant events or as median values with an interquartile range. To identify statistically significant differences between the 2 groups, we performed the Mann-Whitney U-test for continuous variables and the chi-square test for categorical variables. Multivariate logistic regression analysis was used to identify the independent predictors for advanced tumor stage (≥pathological T3 [pT3]). Receiver operating characteristic (ROC) curve analysis was conducted to determine the predictive accuracy of significant predictors of advanced tumor stage. Two-sided p-values <0.005 were considered to indicate statistical significance. IBM SPSS ver. 22.0 (IBM Co., Armonk, NY, USA) and GraphPad Prism software (GraphPad Software Inc., San Diego, CA, USA) were used for all statistical analyses in the present study.
Go to :

RESULTS
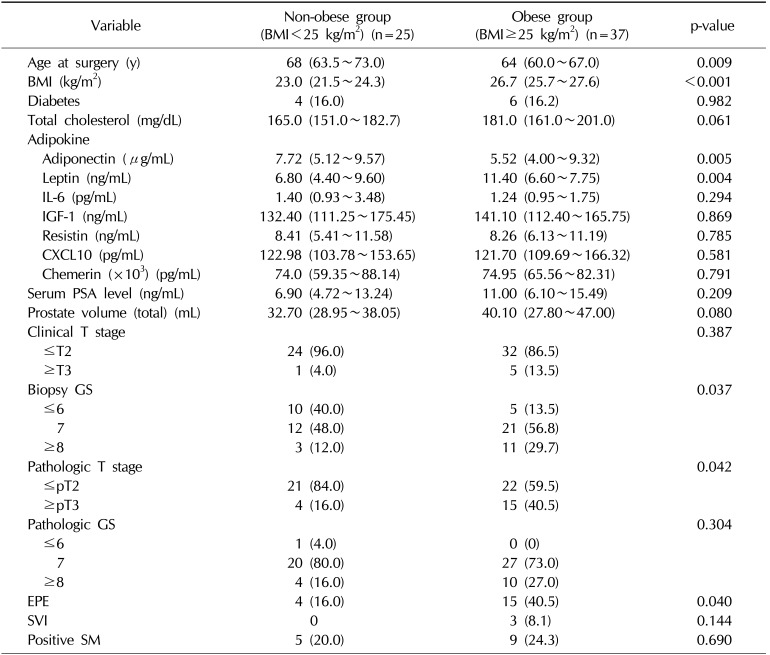
Table 1 shows the baseline characteristics of patients with localized PCa who underwent RP according to preoperative BMI (<25 kg/m
2 or ≥25 kg/m
2). Although the obese patients (BMI ≥25 kg/m
2) were younger than the patients with a lower BMI (<25 kg/m
2), they had a higher proportion of advanced tumors, with higher rates of a GS ≥8, pT3 stage, and positive EPE. Additionally, patients with obesity had significantly lower serum adiponectin (5.52 µg/mL vs. 7.72 µg/mL) and higher leptin levels (11.40 ng/mL vs. 6.80 ng/mL) than those with a normal BMI, as shown in
Fig. 1. However, no significant differences were found between the 2 groups in the levels of the other adipokines that were evaluated, total cholesterol levels, or the proportion of participants with diabetes mellitus (
Fig. 2).
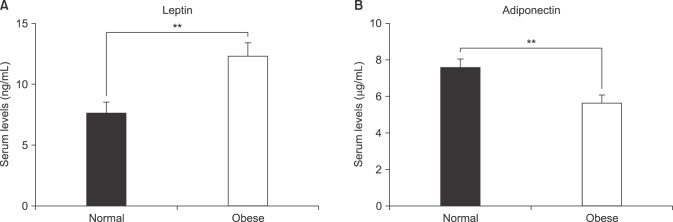 | Fig. 1Comparison of the serum levels of leptin (A) and adiponectin (B) in patients with clinically localized prostate cancer who underwent radical prostatectomy according to their body mass index (non-obese [<25 kg/m2] vs. obese [≥25 kg/m2]). Values of adipokine levels are represented as mean±standard deviation (**p<0.01).
|
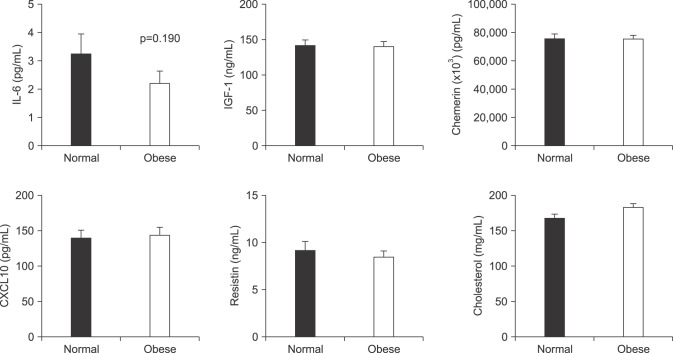 | Fig. 2Comparison of the levels of other circulating adipokines, including IL-6, IGF-1, chemerin, CXCL-10, resistin, and cholesterol in patients with clinically localized prostate cancer who underwent radical prostatectomy according to their body mass index (non-obese [<25 kg/m2] vs. obese [≥25 kg/m2]). Values of adipokine levels are represented as mean±standard deviation. IL: interleukin, IGF: insulin-like growth factor, CXCL: C-X-C motif chemokine.
|
Table 1
Baseline demographics of patients with prostate cancer who underwent radical prostatectomy according to BMI
|
Variable |
Non-obese group (BMI<25 kg/m2) (n=25) |
Obese group (BMI≥25 kg/m2) (n=37) |
p-value |
|
Age at surgery (y) |
68 (63.5~73.0) |
64 (60.0~67.0) |
0.009 |
|
BMI (kg/m2) |
23.0 (21.5~24.3) |
26.7 (25.7~27.6) |
<0.001 |
|
Diabetes |
4 (16.0) |
6 (16.2) |
0.982 |
|
Total cholesterol (mg/dL) |
165.0 (151.0~182.7) |
181.0 (161.0~201.0) |
0.061 |
|
Adipokine |
|
|
|
|
Adiponectin (µg/mL) |
7.72 (5.12~9.57) |
5.52 (4.00~9.32) |
0.005 |
|
Leptin (ng/mL) |
6.80 (4.40~9.60) |
11.40 (6.60~7.75) |
0.004 |
|
IL-6 (pg/mL) |
1.40 (0.93~3.48) |
1.24 (0.95~1.75) |
0.294 |
|
IGF-1 (ng/mL) |
132.40 (111.25~175.45) |
141.10 (112.40~165.75) |
0.869 |
|
Resistin (ng/mL) |
8.41 (5.41~11.58) |
8.26 (6.13~11.19) |
0.785 |
|
CXCL10 (pg/mL) |
122.98 (103.78~153.65) |
121.70 (109.69~166.32) |
0.581 |
|
Chemerin (×103) (pg/mL) |
74.0 (59.35~88.14) |
74.95 (65.56~82.31) |
0.791 |
|
Serum PSA level (ng/mL) |
6.90 (4.72~13.24) |
11.00 (6.10~15.49) |
0.209 |
|
Prostate volume (total) (mL) |
32.70 (28.95~38.05) |
40.10 (27.80~47.00) |
0.080 |
|
Clinical T stage |
|
|
0.387 |
|
≤T2 |
24 (96.0) |
32 (86.5) |
|
≥T3 |
1 (4.0) |
5 (13.5) |
|
Biopsy GS |
|
|
0.037 |
|
≤6 |
10 (40.0) |
5 (13.5) |
|
7 |
12 (48.0) |
21 (56.8) |
|
≥8 |
3 (12.0) |
11 (29.7) |
|
Pathologic T stage |
|
|
0.042 |
|
≤pT2 |
21 (84.0) |
22 (59.5) |
|
≥pT3 |
4 (16.0) |
15 (40.5) |
|
Pathologic GS |
|
|
0.304 |
|
≤6 |
1 (4.0) |
0 (0) |
|
7 |
20 (80.0) |
27 (73.0) |
|
≥8 |
4 (16.0) |
10 (27.0) |
|
EPE |
4 (16.0) |
15 (40.5) |
0.040 |
|
SVI |
0 |
3 (8.1) |
0.144 |
|
Positive SM |
5 (20.0) |
9 (24.3) |
0.690 |

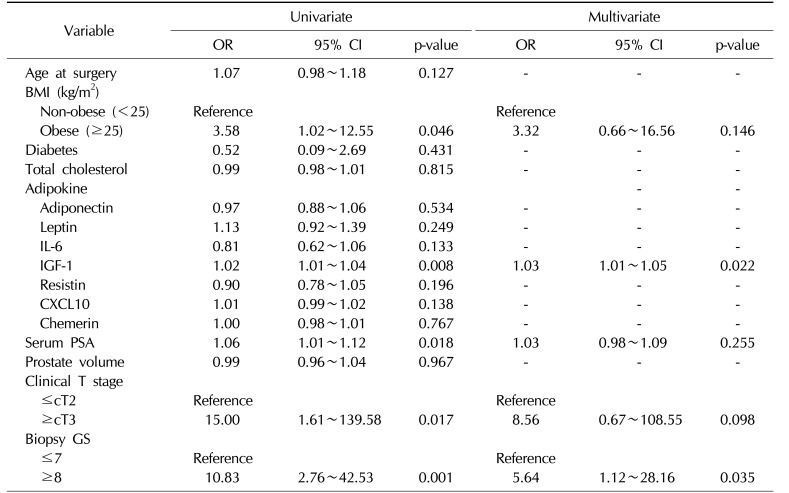
We then investigated whether serum adipokine levels were significant predictive factors for pathological outcomes in the overall population of patients with PCa by performing multivariate analysis. Notably, IGF-1 (odds ratio [OR]=1.03; 95% confidence interval [CI], 1.01~1.05) and a high GS (≥8) (OR=5.64; 95% CI, 1.12~28.16) were identified as predictors of advanced tumor stage (≥pT3) after adjusting for various clinicopathological variables (
Table 2). Furthermore, when we performed multivariate analysis in obese patients with PCa, only leptin remained a significant predictive factor of pathologically advanced tumors (≥pT3) (OR=1.15; 95% CI, 1.01~1.32) (
Table 3). However, no adipokines were identified as predictors of aggressive tumors with a GS ≥8, even in the univariate analysis (data not shown).
Table 2
Multivariate logistic regression models for predicting advanced tumor stage in all patients with prostate cancer who underwent radical prostatectomy
|
Variable |
Univariate |
Multivariate |
|
OR |
95% CI |
p-value |
OR |
95% CI |
p-value |
|
Age at surgery |
1.07 |
0.98~1.18 |
0.127 |
- |
- |
- |
|
BMI (kg/m2) |
|
|
|
|
|
|
|
Non-obese (<25) |
Reference |
|
|
Reference |
|
|
|
Obese (≥25) |
3.58 |
1.02~12.55 |
0.046 |
3.32 |
0.66~16.56 |
0.146 |
|
Diabetes |
0.52 |
0.09~2.69 |
0.431 |
- |
- |
- |
|
Total cholesterol |
0.99 |
0.98~1.01 |
0.815 |
- |
- |
- |
|
Adipokine |
|
|
|
|
|
|
|
Adiponectin |
0.97 |
0.88~1.06 |
0.534 |
- |
- |
- |
|
Leptin |
1.13 |
0.92~1.39 |
0.249 |
- |
- |
- |
|
IL-6 |
0.81 |
0.62~1.06 |
0.133 |
- |
- |
- |
|
IGF-1 |
1.02 |
1.01~1.04 |
0.008 |
1.03 |
1.01~1.05 |
0.022 |
|
Resistin |
0.90 |
0.78~1.05 |
0.196 |
- |
- |
- |
|
CXCL10 |
1.01 |
0.99~1.02 |
0.138 |
- |
- |
- |
|
Chemerin |
1.00 |
0.98~1.01 |
0.767 |
- |
- |
- |
|
Serum PSA |
1.06 |
1.01~1.12 |
0.018 |
1.03 |
0.98~1.09 |
0.255 |
|
Prostate volume |
0.99 |
0.96~1.04 |
0.967 |
- |
- |
- |
|
Clinical T stage |
|
|
|
|
|
|
|
≤cT2 |
Reference |
|
|
Reference |
|
|
|
≥cT3 |
15.00 |
1.61~139.58 |
0.017 |
8.56 |
0.67~108.55 |
0.098 |
|
Biopsy GS |
|
|
|
|
|
|
|
≤7 |
Reference |
|
|
Reference |
|
|
|
≥8 |
10.83 |
2.76~42.53 |
0.001 |
5.64 |
1.12~28.16 |
0.035 |

Table 3
Multivariate logistic regression models for predicting advanced pathological stage in obese patients with a BMI greater than 25 kg/m2 with prostate cancer who underwent radical prostatectomy
|
Variable |
Univariate |
Multivariate |
|
OR |
95% CI |
p-value |
OR |
95% CI |
p-value |
|
Age at surgery |
1.10 |
0.97~1.25 |
0.128 |
- |
- |
- |
|
BMI |
1.29 |
0.76~2.16 |
0.336 |
0.96 |
0.40~2.29 |
0.93 |
|
Diabetes |
0.24 |
0.02~2.32 |
0.220 |
- |
- |
- |
|
Total cholesterol |
1.01 |
0.98~1.02 |
0.780 |
- |
- |
- |
|
Adipokine |
|
|
|
|
|
|
|
Adiponectin |
0.86 |
0.66~1.14 |
0.304 |
- |
- |
- |
|
Leptin |
1.13 |
1.01~1.27 |
0.049 |
1.15 |
1.01~1.32 |
0.045 |
|
IL-6 |
0.87 |
0.64~1.18 |
0.379 |
- |
- |
- |
|
IGF-1 |
1.02 |
0.99~1.03 |
0.135 |
- |
- |
- |
|
Resistin |
0.89 |
0.73~1.09 |
0.264 |
- |
- |
- |
|
CXCL10 |
1.01 |
0.99~1.02 |
0.310 |
- |
- |
- |
|
Chemerin |
1.00 |
1.00~1.00 |
0.194 |
- |
- |
- |
|
Serum PSA |
1.04 |
0.97~1.10 |
0.204 |
1.05 |
0.97~1.14 |
0.212 |
|
Prostate volume |
0.98 |
0.94~1.03 |
0.508 |
|
|
|
|
Clinical T stage |
|
|
|
|
|
|
|
≤cT2 |
Reference |
|
|
Reference |
|
|
|
≥cT3 |
7.64 |
0.76~76.89 |
0.084 |
3.49 |
0.20~60.87 |
0.390 |
|
Biopsy GS |
|
|
|
|
|
|
|
≤7 |
Reference |
|
|
Reference |
|
|
|
≥8 |
3.93 |
0.89~17.36 |
0.070 |
4.25 |
0.82~22.03 |
0.084 |

To evaluate the predictive accuracy of IGF-1 and leptin, we performed an ROC curve analysis of IGF-1 and leptin to assess their ability to discriminate advanced tumor stage (≥pT3) in the overall and obese patient populations, respectively. The area under the curve values of IGF-1 and leptin were 0.751 (p=0.002) and 0.718 (p=0.026) with statistical significance, as shown in
Fig. 3.
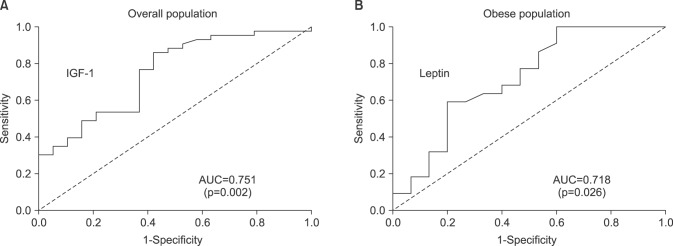 | Fig. 3Receiver operating characteristic curve and corresponding AUC analysis of IGF-1 in the overall population (A) and leptin in the obese population (B), respectively, assessing their ability to discriminate advanced tumor stage (≥pathologic T3) in patients with prostate cancer who underwent radical prostatectomy. IGF: insulin-like growth factor, AUC: area under the curve.
|
Go to :

DISCUSSION
To the best of our knowledge, this is the first prospective study to investigate the profiles of serum adipokines and to identify the predictors of advanced tumor stage in Korean men with localized PCa who underwent RP. Notably, obese patients with PCa had significantly lower serum adiponectin and higher serum leptin levels than those with a normal BMI, but no significant differences were found in the other adipokines that were analyzed. More importantly, while IGF-1 was identified as a significant predictor of advanced tumor stage in the overall population, our study demonstrated that only leptin remained an independent factor for predicting advanced tumor stage in obese patients with PCa. In this regard, we believe that a high leptin level in men with obesity should be considered a significant risk factor for aggressive PCa following surgery.
Recent studies have shown that patients with obesity are at a higher risk of developing cancer and have worse survival outcomes than those without obesity [
11]. Regarding the potential underlying mechanisms of the relationship between obesity and tumor biology, several factors have been suggested, including enhanced secretion of steroid hormones, chronic insulin resistance, and a sub-chronic inflammatory state [
2]. Obesity is also associated with an increased risk of PCa, particularly for high-risk or advanced-stage tumors, as well as an increased risk of disease recurrence [
12,
13]. Additionally, higher BMI is associated with a higher risk of upstaging and upgrading in patients with low-risk PCa [
14]. Consistent with these findings, our data showed that obese patients with a BMI greater than 25 kg/m
2 had a higher proportion of tumors with a high GS (≥8), pT3 stage, and positive EPE than those with a BMI less than 25 kg/m
2.
Mechanistically, there are 3 potential molecular pathways linking obesity and advanced-stage PCa: the IGF-1 signaling pathway, the sex hormone axis, and various adipokines [
15]. Adipokines are of significant research interest because they directly influence cancer cells via the bloodstream; furthermore, they can be used as simple and useful biomarkers for predicting disease status [
7]. In addition to the endocrine effects of circulating adipokines on target cancer cells, paracrine effects are also crucial to the progression of PCa, particularly in the case of capsular invasion or periprostatic fat invasion [
7]. Through these 2 pathways, the malignant cells of the prostate gland are persistently exposed to increased levels of various adipokines, which act as anti-apoptotic, proliferative, and angiogenic factors, potentiating their ability for proliferation and progression [
9].
In our study, leptin was identified as a significant predictive factor of advanced PCa in obese patients, which is consistent with previous studies demonstrating that leptin was associated with tumorigenesis and progression of PCa through its mediation of key oncogenic signaling molecules [
16]. Iwamoto and colleagues showed that leptin promoted cell proliferation by inducing the JNK pathway in human PCa cells (DU145 and PC-3) [
17]. In a study by Somasundar et al [
18], leptin inhibited tumor cell apoptosis by activating the AKT signaling pathway in DU145 cells, and led to enhanced cell growth and survival via upregulation of the ERK signaling pathway in PC-3 cells. Moreover, leptin significantly enhanced cellular migration by activating the expression of various growth factors for angiogenesis and the epithelial-mesenchymal transition, such as vascular endothelial growth factor (VEGF) and transforming growth factor-β1, in androgen-independent PCa cells [
19]. Therefore, leptin can be regarded as an adipokine that leads to negative outcomes in obese patients with PCa who undergo surgery, but not in patients with a normal BMI.
Several limitations of the current study should be acknowledged. First, the small number of patients evaluated is a crucial drawback despite the prospective nature of our study, and therefore our conclusions cannot be generalized to all obese patients with localized PCa based on the results of this study alone. Second, some well-known prognostic factors of PCa, such as preoperative PSA, biopsy GS, and clinical T stage, were not identified as independent predictors of advanced PCa in the multivariate analysis. We note that the present study only included clinically localized cases of PCa treated with surgery with similar clinicopathological characteristics, and we speculate that this selection bias may have influenced the final statistical outcomes. Third, although GS is the most critical factor for the prognosis of PCa, we were not able to identify adipokines that showed a statistically significant ability to predict high GS (≥8) in the univariate or multivariate analysis. We hope to identify which serum adipokines are useful for predicting higher GS in a follow-up study with a larger study population size to ensure the clinical significance of these novel biomarkers. Fourth, we did not measure lipid profile parameters, including low-density lipoprotein, high-density lipoprotein, and triglycerides, as well as other potential adipokines, such as IL-2, VEGF, and TNF-α. Fifth, we measured circulating adipokine levels at a single time point with biological duplication. To improve the quality of the data, we should perform repeated measurements and compare the levels of each adipokine through an internal validation study. Sixth, we were not able to determine whether adipokines predicted biochemical recurrence because few recurrence events (n=6) took place during the short-term study period. Since the prediction of disease recurrence using simple biomarkers is of great clinical importance, an extended analysis should be carried out with a long-term follow-up period in the future. Finally, the key results of this study were based on a single-center database, and therefore an external validation study should be performed to verify our findings.
Go to :

CONCLUSIONS
In summary, obese patients (BMI ≥25 kg/m2) with PCa who underwent RP had a higher proportion of tumors with high GS (≥8), pT3 stage, and positive EPE, as well as lower serum adiponectin and higher serum leptin levels than patients with a normal BMI, but they did not show differences in the other adipokines that were evaluated. Furthermore, leptin was identified as a significant predictor of advanced tumor stage in obese patients with PCa, indicating that a higher leptin level in men with obesity can be considered a risk factor for aggressive PCa. Our prospective study provides insights into the role of circulating adipokines in Korean patients with PCa undergoing RP, particularly in patients with obesity.
Go to :











 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download