Abstract
Prostatic calculi often occur in middle-aged and old men. Prostatic calculi are usually classified as primary/endogenous stones or secondary/extrinsic stones. Endogenous stones are commonly caused by obstruction of the prostatic ducts around the enlarged prostate by benign prostatic hyperplasia (BPH) or by chronic inflammation. Extrinsic stones occur mainly around the urethra, because they are caused by urine reflux. The exact prevalence of prostatic calculi is not known, and it has been reported to vary widely, from 7% to 70%. Most cases of prostatic calculi are not accompanied by symptoms. Therefore, most cases are found incidentally during the diagnosis of BPH using transrectal ultrasonography (TRUS). However, prostatic calculi associated with chronic prostatitis may be accompanied by chronic pelvic pain. Rare cases have been reported in which extrinsic prostatic calculi caused by urine reflux have led to voiding difficulty due to their size. More than 80% of prostatic calculi are composed of calcium phosphate. Prostatic calculi can be easily diagnosed using TRUS or computed tomography. Treatment is often unnecessary, but if an individual experiences difficulty in urination or chronic pain, prostatic calculi can be easily removed using a transurethral electroresection loop or holmium laser.
Transrectal ultrasonography (TRUS) is used to diagnose benign prostatic hyperplasia in an increasing number of patients. As TRUS has become more common, benign prostatic hyperplasia has often been found to be accompanied by prostatic calculi. However, few studies have been conducted on prostatic calculi. In 1586, Donatus reported them for the first time in an autopsy. The first clinical report of urinary symptoms associated with stones was published in the late 19th century [1]. To date, it has been accepted that prostatic calculi occur in proportion to age without causing any specific symptoms [2]. Recently, as the implementation of TRUS has increased, more research on prostatic calculi has been conducted, and there have been some reports on the shape and composition of the stones. However, the incidence of stones, the mechanism of their formation, their relationship to benign or malignant findings in the prostate, and the clinical significance of stones are not yet known.
The prostate is a male reproductive organ composed of glandular tissue and the fibrous tissue surrounding it. The average prostate weight of a normal adult is about 20 g. The length of the long axis is 4 cm, the width is 2 cm, and the length is 2 cm. The prostate completely surrounds the prostatic urethra, and there are 15 to 30 conduits opening from the prostate urethra. The entire prostate is surrounded by a connective tissue layer rich in plexus and elastic fibers, and the hull is surrounded by prostatic interstitial tissue. The prostate is attached to the bladder neck on the back on the upper side, is fixed to the anterior labral prostate ligament, and is fixed by the urogenital septum on the lower side. Behind the prostate is the Denonvilliers fascia, which separates this region from the rectum. The anterior and lateral segments are attached to the pelvic floor and wrapped in the endopelvic fascia. The prostatic urethra and verumontanum are in the prostate, and 2 posterior ejaculatory ducts enter the prostate urethra [2]. In 1912, Lowsley divided the prostate into 5 lobes, comprising 2 lateral lobes, 1 anterior lobe, 1 middle lobe, and 1 posterior lobe. However, this concept of a lobular structure has evolved into a zonal concept, as articulated by McNeal in 1968. The prostate gland tissue is divided into 5 zones: the central zone, the peripheral zone, the transitional zone, the anterior fibromuscular stroma, and the preprostatic sphincter, according to the location of the urethra, distribution of pathologic lesions, and patterns of development (Fig. 1) [3]. Each site can be investigated by TRUS; this classification is consistent with the anatomical structure of the prostate, and is also recognized as clinically useful in that it is consistent with differences in the sites of the major diseases that occur in the prostate. The inferior oblique ganglion passes through the entire prostatic sphincter just beneath the lateral and posterior sides and normally occupies 5% to 10% of the tissue of the prostate gland. The fibrous band separates the transition zone from the surrounding glandular tissue, and this muscle band can also be observed using TRUS. Benign prostatic hyperplasia occurs most often in the transitional zone, and 20% of prostate cancers occur at this site.
The tube system of the central zone occurs around the ejaculatory duct of the prostate and accounts for 25% of the tissue of the gland; 1% to 5% of prostate cancer cases occur at this site. The peripheral zone accounts for almost 70% of the tissue of the prostate gland in the posterior and lateral regions. Seventy percent of cases of prostate cancer occur in this area, and it is also the site where chronic prostatitis occurs. Fibromuscular stroma predominates in up to 33% of prostates, and invasion of this tissue by cancer is rare. Prostatic fluid is discharged through the tube system to the urethra. The prostate tube system is an independent functional unit of the prostate, and the secreted fluid is discharged to the urethra through the respective draining tube. The tube system is arranged concentrically around the urethra, and when viewed from the urethral opening, appears to extend like tree branches. The human prostate has more than 30 tubular structures and is open to the prostate urethra. Prostatic calculi can occur all over the prostate in the case of multiple prostatic calculi. However, benign prostatic hyperplasia is often caused by blockage of the glandular tissue around the capsules of hypertrophic prostate adenomas due to the presence of benign prostatic hyperplasia in the transition zone [4]. Therefore, in patients with benign prostatic hyperplasia, the resection margin or extent of transurethral resection of the prostate (TURP) may be restricted to the site where the prostate calculi are visible (Fig. 1).
Prostatic calculi can be divided into primary/endogenous stones (occurring within the acini of the prostate) (Fig. 2A) or secondary/extrinsic (caused by reflux of urine into the prostate) (Fig. 2B) [5]. However, the term ‘prostatic calculus,’ strictly speaking, means only a primary/endogenous prostatic calculus. Klimas et al [1] suggested that prostatic secretions, corpora amylacea, or inflammation of the prostate can block the secretory tube, leading to thickening (Fig. 3) and calcification of the stones. Stones exist in the form of several small stones, ranging from 0.5 to 5.0 mm in size. Stones are a pathophysiologic phenomenon occurring during the aging process, mainly after age 50 years. Moore [6] and Kirby et al [7], as part of their proposed hypothesis regarding extrinsic stones, concluded that prostatic hypertrophy causes chronic prostate inflammation, which affects the formation of calculi.
Endogenous prostatic calculi are mainly found in the head (front) part of the posterior lobe and in the large tube and acini of the lateral lobe of the prostate. The calculi in the acini are fine, whereas those inside the tube are large and easily visible. Because extrinsic prostatic calculi are mainly caused by urinary reflux into the prostate, they are fewer in number than endogenous prostate calculi, but are often larger. The age of onset of extrinsic prostatic calculi varies, and they are often secondary to neurogenic bladder or chronic urinary tract infection rather than age. In severe cases, prostate enlargement has been reported to cause urethral closure [8]. However, despite this hypothesis, the precise mechanism of development of prostatic calculi is still unclear.
There are few studies and therefore insufficient data on the components of prostatic calculi. Recently, studies analyzing the components of prostatic calculi were published by Sfanos et al [9] and Dessombz et al [10]. Sfanos et al [9] showed that calcium phosphate accounted for 82.6% (19 of 23) of prostatic calculi. Calcium carbonate phosphate accounted for 8.7% (2 of 23), calcium oxalate monohydrate accounted for 4.4% (1 of 23), and calcium phosphate and calcium oxalate monohydrate-mixed calculi accounted for 4.4% (Table 1). Dessombz et al [10] reported that a combination of calcium phosphate, calcium phosphate, and calcium carbonate phosphate was the most abundant composition found in 23 prostatic calculi.
Prostatic calculi are a very common finding, but it is difficult to know the exact frequency of this finding, and the prevalence varies across reports (Table 2) [111213141516]. Harada et al [17] reported that calculi were observed in 68.8% of patients with benign prostatic hyperplasia, and Kim et al [18] reported that calculi were observed in 70% of elderly patients with benign prostatic hyperplasia. Lee et al [19] reported that calculi were observed in 40.7% of patients when an ultrasonographic examination was performed, regardless of age. Shoskes et al [15] reported that 46.8% of patients with chronic pelvic pain had prostatic calculi, excluding calculi less than 3 mm in diameter, and Geramoutsos et al [16] reported a prevalence of 7.4%. Therefore, the prevalence of prostatic calculi in patients with benign prostatic hyperplasia or prostatitis is greater than in those with a normal prostate.
Prostatic calculi associated with simple benign prostatic hyperplasia are often asymptomatic. Prostatic calculi associated with chronic prostatitis may be closely related to lower urinary tract symptoms. Leader and Queen [20] pointed out that small prostatic calculi are clinically meaningless and occur physiologically in the process of aging. Søndergaard et al [21] also reported that prostatic calculi are part of the normal aging process and have little clinical significance. In that report, prostatic calculi were not described as causing clinical symptoms, and, even if they did, the authors suggested that prostatic calculi can be thought of as a symptom of underlying disease, such as benign prostatic hyperplasia. However, chronic prostate inflammatory conditions other than simple benign prostatic hyperplasia may be the source of persistent inflammation in patients with pelvic pain. In such patients, prostatic calculi not only serve as a site for infectious bacteria to colonize, but also promote the closure of the secretory tubes of the prostate, which may cause resistance to antibiotics and other medications. These conclusions are supported by the findings of Shoskes et al [15] that prostatic calculi in patients with chronic pelvic pain syndrome were associated with inflammation, bacterial colonization, and symptom duration. Geramoutsos et al [16] reported that small prostatic calculi were normal as subjects grow older, but that larger calculi were associated with chronic prostate inflammation and lower urinary tract symptoms. Geramoutsos et al [16] classified calculi into a group around the center/ urethra and a group at the periphery of the urethra. Kim et al [18] did not observe any differences in the storage and urination symptoms of the International Prostate Symptom Score according to the type and location of prostatic calculi and the presence of lower urinary tract symptoms. However, Cha et al [22] reported that when prostate calculi were present in the periurethral prostate transit zone, they may have caused lower urinary tract symptoms to worsen, and a case of urinary retention due to a massive prostatic calculus has also been reported.
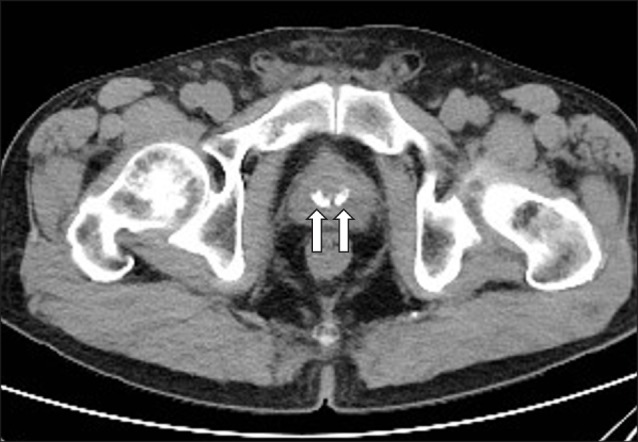
Prostatic calculi are mainly found using TRUS (Fig. 3) in the process of diagnosing lower urinary tract symptoms. Harada et al [17] divided patients into 2 groups according to the echo patterns of prostatic calculi: (1) type A: discrete, multiple small echoes, usually diffusely distributed throughout the gland and (2) type B: a large mass of multiple, coarser echoes. In addition to TRUS, a computed tomography (CT) scan (Fig. 4) or a kidney, ureter, and bladder x-ray may find prostatic calculi incidentally, but in most cases, finding prostate calculi is not the goal; thus, even if they are diagnosed, they are mostly clinically meaningless. Fig. 4 and 5 are TRUS and CT images, respectively, of the same patient.
Prostatic calculi are mostly asymptomatic, but in some cases, a large prostatic calculus protruding into the urethra causes severe lower urinary tract symptoms such as urinary obstruction. In such cases, the prostatic calculi can be removed with a transurethral endoscope. Prostatic calculi associated with benign prostatic hyperplasia tend to occur in adjacent areas of the glomeruli, as the compressed prostate ducts surrounding the glandular mass are occluded. Prostatic calculi observed on the border of enlarged prostate tissue during TURP also act as a boundary for prostate capsules during TURP, and can be easily removed by endoscopic resection. In a study of 183 patients with benign prostatic hyperplasia who underwent TURP, Jeon et al [23] reported that the group with prostatic calculi showed a greater improvement in lower urinary tract symptoms after TURP than the group without prostatic calculi. For calculi around the urethra (between the periurethral prostatic gland and the urethra) and around the adenoma (between the adenoma and the prostate tissue), it was shown that the greater the number of prostate calculi removed, the more significant was the change in lower urinary tract symptoms. However, calculi were not associated with any difference in patients with diffuse scattering in the central or peripheral region. Multiple calculi in the peripheral zone can be difficult to remove entirely with these surgical treatments.
Prostatic calculi, which are usually symptomless, generally require no special treatment. However, the most problematic cases of prostatic calculi are associated with chronic prostate inflammation. In this case, treatment with antibiotics combined with the treatment of prostatitis may cause the symptoms to disappear. However, because prostate calculi contaminated with bacteria are a source of persistent inflammation, the thorough elimination of prostate calculi is the preferred treatment method for chronic bacterial prostate inflammation. Lee and Kim [24] analyzed the efficacy of oral antibiotics in 64 patients with chronic bacterial prostatitis and reported that the healing rate using pharmacotherapy was 63.6% in patients without calculi and 35.7% in those with calculi; they emphasized the usefulness of TURP for the treatment of chronic bacterial prostate inflammation rather than relying on medical treatment.
As the use of TRUS in patients with benign prostatic hyperplasia is increasing, prostatic calculi are more commonly found. However, little is known about the incidence of calculi in the prostate, their mechanism of formation, their associations with benign or malignant prostate findings, and the clinical significance of the calculi.
The results of the present study suggest that prostatic calculi may be caused by the obstruction of prostate secretions around enlarged tissues or occlusion by chronic inflammation via benign prostatic hyperplasia. The incidence of prostatic calculi has been reported to vary widely (from 7% to 70%) across reports. Prostatic calculi are rarely accompanied by symptoms; hence, they are often found incidentally during the diagnosis of benign prostatic hyperplasia using transurethral ultrasonography. However, prostatic calculi associated with chronic prostate inflammation may be accompanied by chronic pelvic pain and, in the case of extrinsic prostatic calculi, have been reported to cause voiding dysfunction due to their large size in some rare cases. More than 80% of prostatic calculi are composed of calcium phosphate. Prostatic calculi can be easily diagnosed using TRUS and CT. Treatment is not usually necessary, but prostatic calculi can be easily removed with a transurethral electroresection loop or holmium laser if they cause difficulty in urination or chronic pain.
References
1. Klimas R, Bennett B, Gardner WA Jr. Prostatic calculi: a review. Prostate. 1985; 7:91–96. PMID: 3909127.

2. Lee CH, Akin-Olugbade O, Kirschenbaum A. Overview of prostate anatomy, histology, and pathology. Endocrinol Metab Clin North Am. 2011; 40:565–575. PMID: 21889721.

5. McDonald HP, Upchurch WE, Sturdevant CE. Treatment of prostatic calculi. JAMA. 1955; 157:787–788.

6. Moore RA. Morphology of prostatic corpora amylacea and calculi. Arch Pathol. 1936; 22:22–40.
7. Kirby RS, Lowe D, Bultitude MI, Shuttleworth KE. Intra-prostatic urinary reflux: an aetiological factor in abacterial prostatitis. Br J Urol. 1982; 54:729–731. PMID: 7150931.

8. Najoui M, Qarro A, Ammani A, Alami M. Giant prostatic calculi. Pan Afr Med J. 2013; 14:69. PMID: 23565316.

9. Sfanos KS, Wilson BA, De Marzo AM, Isaacs WB. Acute inflammatory proteins constitute the organic matrix of prostatic corpora amylacea and calculi in men with prostate cancer. Proc Natl Acad Sci U S A. 2009; 106:3443–3448. PMID: 19202053.

10. Dessombz A, Méria P, Bazin D, Daudon M. Prostatic stones: evidence of a specific chemistry related to infection and presence of bacterial imprints. PLoS One. 2012; 7:e51691. PMID: 23272143.

11. Dell'Atti L, Galosi AB, Ippolito C. Prostatic calculi detected in peripheral zone of the gland during a transrectal ultra sound biopsy can be significant predictors of prostate cancer. Arch Ital Urol Androl. 2016; 88:304–307. PMID: 28073198.
12. Park B, Choo SH. The burden of prostatic calculi is more important than the presence. Asian J Androl. 2017; 19:482–485. PMID: 27184549.

13. Hong CG, Yoon BI, Choe HS, Ha US, Sohn DW, Cho YH. The prevalence and characteristic differences in prostatic calcification between health promotion center and urology department outpatients. Korean J Urol. 2012; 53:330–334. PMID: 22670192.

14. Kim WB, Doo SW, Yang WJ, Song YS. Influence of prostatic calculi on lower urinary tract symptoms in middle-aged men. Urology. 2011; 78:447–449. PMID: 21689847.

15. Shoskes DA, Lee CT, Murphy D, Kefer J, Wood HM. Incidence and significance of prostatic stones in men with chronic prostatitis/chronic pelvic pain syndrome. Urology. 2007; 70:235–238. PMID: 17826477.

16. Geramoutsos I, Gyftopoulos K, Perimenis P, Thanou V, Liagka D, Siamblis D, et al. Clinical correlation of prostatic lithiasis with chronic pelvic pain syndromes in young adults. Eur Urol. 2004; 45:333–337. PMID: 15036679.

17. Harada K, Igari D, Tanahashi Y. Gray scale transrectal ultransonography of the prostate. J Clin Ultrasound. 1979; 7:45–49. PMID: 108298.
18. Kim SH, Jung KI, Lee BH, Lee BY, Cho SY, Kim HW. Relations between prostatic calculi and lower urinary tract symptoms of benign prostatic hyperplasia. J Korean Continence Soc. 2009; 13(1):30–36.

19. Lee SE, Ku JH, Park HK, Jeong CK, Kim SH. Prostatic calculi do not influence the level of serum prostate specific antigen in men without clinically detectable prostate cancer or prostatitis. J Urol. 2003; 170:745–748. PMID: 12913688.

21. Søndergaard G, Vetner M, Christensen PO. Prostatic calculi. Acta Pathol Microbiol Immunol Scand A. 1987; 95:141–145. PMID: 2440234.
22. Cha WH, Kim KH, Seo YJ. The effect of periurethral prostatic calculi on lower urinary tract symptoms in benign prostatic hyperplasia. Korean J Urol. 2008; 49:237–241.

23. Jeon HJ, Chung HC, Song JM. Effects of residual prostatic calculi on lower urinary tract symptoms after transurethral resection of prostate. Korean J Urol. 2005; 46:569–573.
24. Lee BE, Kim SK. The effects of concomitant prostatic calculi to the therapeutic results in patients with chronic bacterial prostatitis. Korean J Urol. 1989; 30:876–884.
Fig. 1
Zonal classification of the prostate and prostatic calculi. AFS: anterior fibromuscular stroma, TZ: transitional zone, CZ: central zone, PZ: peripheral zone.
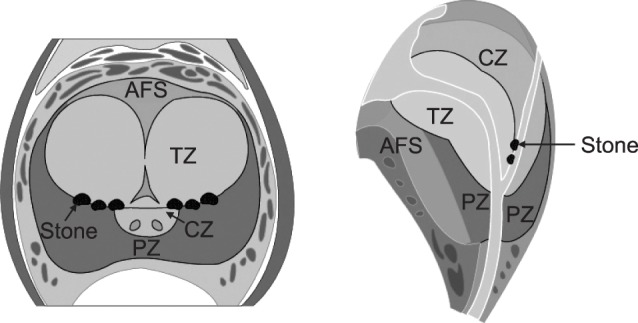
Fig. 2
(A) Primary/endogenous prostatic calculi. (B) Secondary/exogenous prostatic calculi. Arrows indicate prostatic calculi.
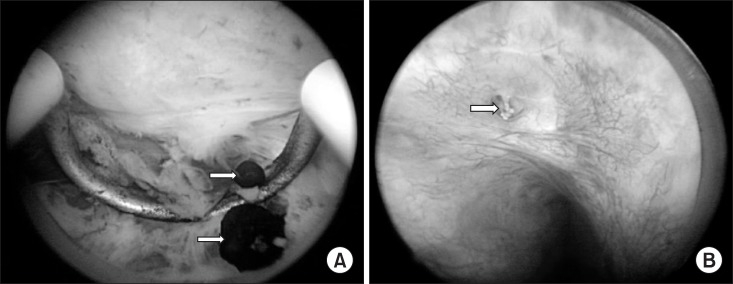
Fig. 3
The solidified dark secretion of the prostate can be seen during holmium laser enucleation of the prostate (arrow).
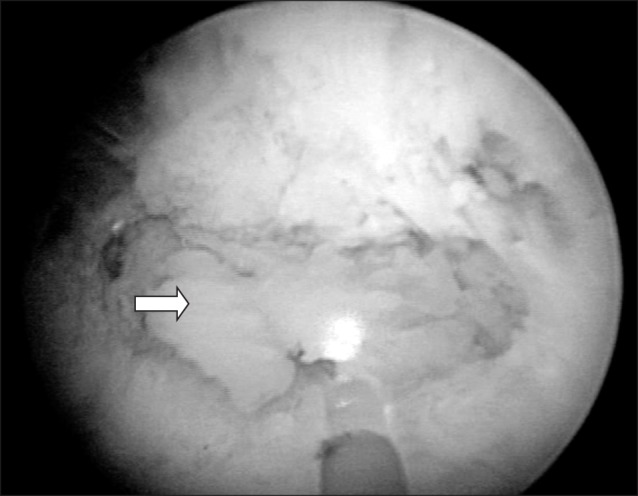
Fig. 4
Transrectal ultrasonography. (A) Transverse view. (B) Sagittal view. Arrows indicate prostatic calculi.
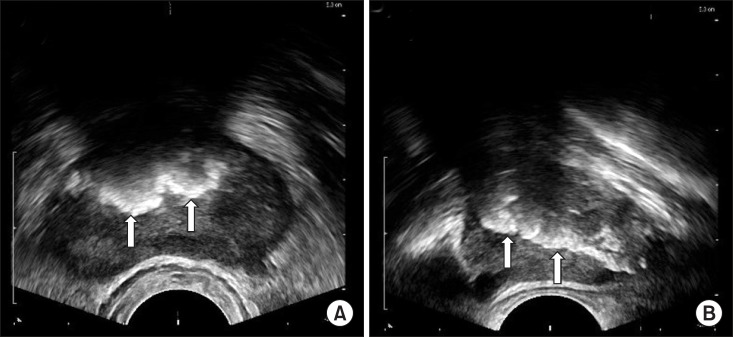
Table 1
The main composition of prostatic calculi
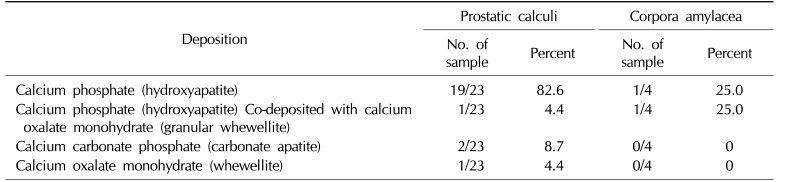
Data from Sfanos et al. Proc Natl Acad Sci USA 2009;106:3443-8) [9].
Table 2
The prevalence and location of prostatic calculi
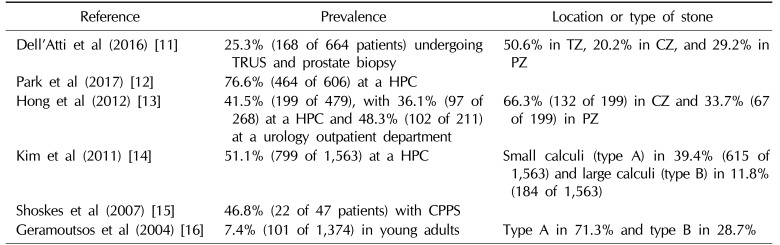
| Reference | Prevalence | Location or type of stone |
|---|---|---|
| Dell'Atti et al (2016) [11] | 25.3% (168 of 664 patients) undergoing TRUS and prostate biopsy | 50.6% in TZ, 20.2% in CZ, and 29.2% in PZ |
| Park and Choo (2017) [12] | 76.6% (464 of 606) at a HPC | |
| Hong et al (2012) [13] | 41.5% (199 of 479), with 36.1% (97 of 268) at a HPC and 48.3% (102 of 211) at a urology outpatient department | 66.3% (132 of 199) in CZ and 33.7% (67 of 199) in PZ |
| Kim et al (2011) [14] | 51.1% (799 of 1,563) at a HPC | Small calculi (type A) in 39.4% (615 of 1,563) and large calculi (type B) in 11.8% (184 of 1,563) |
| Shoskes et al (2007) [15] | 46.8% (22 of 47 patients) with CPPS | |
| Geramoutsos et al (2004) [16] | 7.4% (101 of 1,374) in young adults | Type A in 71.3% and type B in 28.7% |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download