1. Gholami SS, Gonzalez-Cadavid NF, Lin CS, Rajfer J, Lue TF. Peyronie's disease: a review. J Urol. 2003; 169:1234–1241.

2. Hellstrom WJ, Bivalacqua TJ. Peyronie's disease: etiology, medical, and surgical therapy. J Androl. 2000; 21:347–354.
3. Devine CJ Jr, Somers KD, Jordan SG, Schlossberg SM. Proposal: trauma as the cause of the Peyronie's lesion. J Urol. 1997; 157:285–290.

4. Jarow JP, Lowe FC. Penile trauma: an etiologic factor in Peyronie's disease and erectile dysfunction. J Urol. 1997; 158:1388–1390.

5. Gabrielson AT, Alzweri LM, Hellstrom WJ. Collagenase clostridium histolyticum in the treatment of Peyronie's disease: review of a minimally invasive treatment option. World J Mens Health. 2017; 35:134–145.

6. Joice GA, Burnett AL. Nonsurgical interventions for Peyronie's disease: update as of 2016. World J Mens Health. 2016; 34:65–72.

7. Segal RL, Burnett AL. Surgical management for Peyronie's disease. World J Mens Health. 2013; 31:1–11.

8. McKinsey TA. Targeting inflammation in heart failure with histone deacetylase inhibitors. Mol Med. 2011; 17:434–441.

9. Pang M, Zhuang S. Histone deacetylase: a potential therapeutic target for fibrotic disorders. J Pharmacol Exp Ther. 2010; 335:266–272.

10. Huber LC, Distler JH, Moritz F, Hemmatazad H, Hauser T, Michel BA, et al. Trichostatin A prevents the accumulation of extracellular matrix in a mouse model of bleomycin-induced skin fibrosis. Arthritis Rheum. 2007; 56:2755–2764.

11. Hemmatazad H, Rodrigues HM, Maurer B, Brentano F, Pileckyte M, Distler JH, et al. Histone deacetylase 7, a potential target for the antifibrotic treatment of systemic sclerosis. Arthritis Rheum. 2009; 60:1519–1529.

12. Piao S, Choi MJ, Tumurbaatar M, Kim WJ, Jin HR, Shin SH, et al. Transforming growth factor (TGF)-β type I receptor kinase (ALK5) inhibitor alleviates profibrotic TGF-β1 responses in fibroblasts derived from Peyronie's plaque. J Sex Med. 2010; 7:3385–3395.

13. Ryu JK, Kim WJ, Choi MJ, Park JM, Song KM, Kwon MH, et al. Inhibition of histone deacetylase 2 mitigates profibrotic TGF-β1 responses in fibroblasts derived from Peyronie's plaque. Asian J Androl. 2013; 15:640–645.

14. Hoodless PA, Haerry T, Abdollah S, Stapleton M, O'Connor MB, Attisano L, et al. MADR1, a MAD-related protein that functions in BMP2 signaling pathways. Cell. 1996; 85:489–500.

15. Korfei M, Skwarna S, Henneke I, MacKenzie B, Klymenko O, Saito S, et al. Aberrant expression and activity of histone deacetylases in sporadic idiopathic pulmonary fibrosis. Thorax. 2015; 70:1022–1032.

16. Hutt DM, Herman D, Rodrigues AP, Noel S, Pilewski JM, Matteson J, et al. Reduced histone deacetylase 7 activity restores function to misfolded CFTR in cystic fibrosis. Nat Chem Biol. 2010; 6:25–33.

17. Pannem RR, Dorn C, Hellerbrand C, Massoumi R. Cylindromatosis gene CYLD regulates hepatocyte growth factor expression in hepatic stellate cells through interaction with histone deacetylase 7. Hepatology. 2014; 60:1066–1081.

18. Glenisson W, Castronovo V, Waltregny D. Histone deacetylase 4 is required for TGFbeta1-induced myofibroblastic differentiation. Biochim Biophys Acta. 2007; 1773:1572–1582.
19. Guo W, Shan B, Klingsberg RC, Qin X, Lasky JA. Abrogation of TGF-beta1-induced fibroblast-myofibroblast differentiation by histone deacetylase inhibition. Am J Physiol Lung Cell Mol Physiol. 2009; 297:L864–L870.
20. Noh H, Oh EY, Seo JY, Yu MR, Kim YO, Ha H, et al. Histone deacetylase-2 is a key regulator of diabetes- and transforming growth factor-beta1-induced renal injury. Am J Physiol Renal Physiol. 2009; 297:F729–F739.
21. Pang M, Kothapally J, Mao H, Tolbert E, Ponnusamy M, Chin YE, et al. Inhibition of histone deacetylase activity attenuates renal fibroblast activation and interstitial fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol. 2009; 297:F996–F1005.

22. El-Sakka AI, Hassoba HM, Pillarisetty RJ, Dahiya R, Lue TF. Peyronie's disease is associated with an increase in transforming growth factor-beta protein expression. J Urol. 1997; 158:1391–1394.

23. Haag SM, Hauck EW, Szardening-Kirchner C, Diemer T, Cha ES, Weidner W, et al. Alterations in the transforming growth factor (TGF)-beta pathway as a potential factor in the pathogenesis of Peyronie's disease. Eur Urol. 2007; 51:255–261.
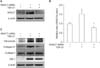
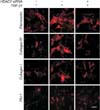
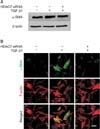
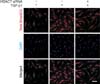
24. Kwon KD, Choi MJ, Park JM, Song KM, Kwon MH, Batbold D, et al. Silencing histone deacetylase 2 using small hairpin RNA induces regression of fibrotic plaque in a rat model of Peyronie's disease. BJU Int. 2014; 114:926–936.







 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download