INTRODUCTION
The organic causes of erectile dysfunction (ED) include arterial, cavernosal, hormonal, and neurological causes [
1]. Arterial ED is associated with atherosclerosis, hyperlipidemia, and hypertension, while cavernosal ED is caused by degenerative changes in the tunica albuginea, degeneration of fibroelastic tissues, and insufficient trabecular smooth muscle relaxation [
234]. Hormonal ED may occur in patients with insufficient testosterone or hyperprolactinemia; additionally, alcohol can affect the hypothalamicpituitary-gonadal hormone axis, decreasing testosterone secretion and potentially causing hormone-induced ED [
56]. Neurological ED occurs due to central nervous system diseases in the brain and spine, as well as insufficient secretion of neurotransmitters such as acetylcholine and nitric oxide (NO) in components of the peripheral nerve system such as the pudendal or cavernosal nerve [
7].
It is known that moderate alcohol consumption increases blood circulation and affects erectile function [
8], while chronic alcohol consumption negatively affects erectile function. However, alcohol-induced sexual dysfunction has so far been primarily analyzed as being due to abnormal testosterone levels [
9], and the mechanism of how alcohol may directly affect the corpus cavernosum (CC) has not yet been elucidated. This study investigated how alcohol affected CC smooth muscle through an organ bath study of alcohol-administered rabbits and how alcohol affected the CC of rats that received acute and chronic administrations of alcohol.
Go to :

DISCUSSION
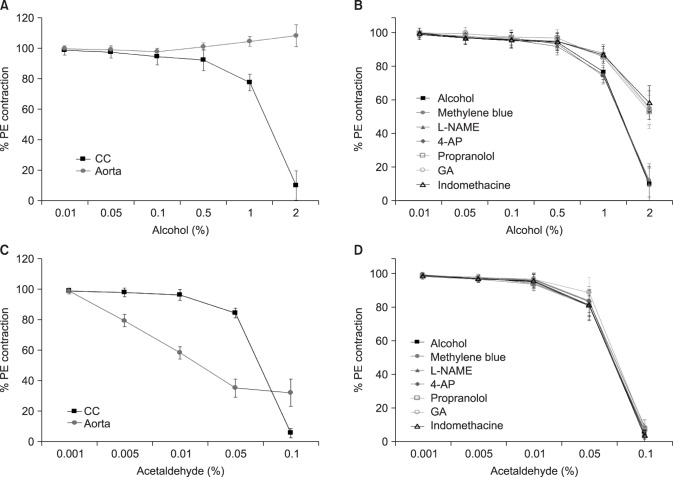
Alcohol caused dose-dependent CC relaxation in the CC smooth muscle of rats. However, it led to contraction at high concentrations in the aorta. This confirms that alcohol led to the smooth muscle contraction in the systemic response to a certain extent, but was associated with relaxation in the CC.
Contraction in the CC takes place if adrenergic α-receptors are activated, while relaxation occurs if adrenergic β-receptors are activated [
101112]. In our study, the effect of alcohol on CC relaxation was significantly inhibited by pretreatment with propranolol, which is an adrenergic β-receptor blocker, and we therefore suggest that acute alcohol administration caused CC relaxation through the activation of β-receptors.
Arachidonic acid is degraded to prostaglandins by cyclooxygenase in CC smooth muscle, affecting the mechanism of erection through various pathways. Prostaglandin inhibits norepinephrine secretion from the adrenergic nerves in the penis and increases the intracellular cAMP concentration in CC smooth muscle [
1314]. This process reduces the intracellular Ca
2+ concentration and leads to erection by enhancing smooth muscle relaxation. Our study showed that the effect of alcohol on CC relaxation was significantly inhibited by pretreatment with indomethacin, which is a cyclooxygenase inhibitor, and we therefore suggest that acute alcohol administration led to CC relaxation through the activation of cyclooxygenase.
K
+ channels play a role in adjusting the tone of CC smooth muscle [
1516]. Hyperpolarization occurs if the K
+ channels are activated and opened, which inhibits Ca
2+ channel activation, reduces the intracellular Ca
2+ concentration, and causes CC smooth muscle relaxation [
1718]. Our study found that the effect of alcohol on CC relaxation was significantly inhibited by pretreatment with glibenclamide, which is a K
ATP channel inhibitor, and 4-aminopyridine, which is a membrane potential-dependent K
+ channel inhibitor, and we therefore suggest that acute alcohol administration led to CC relaxation through activation of K
+ channels.
NO is important in the instantaneous relaxation of the cavernosal vessel and CC smooth muscle. NO is secreted as a result of eNOS activation in CC endothelial cells and by non-adrenergic, non-cholinergic neurotransmission at the cavernosal nerve terminal. NO activates guanylyl cyclase and increases the conversion of guanosine triphosphate transaminase to cGMP, which reduces intracellular Ca
2+ through a complicated process and causes an erection by smooth muscle relaxation [
1920]. In our study, the effect of alcohol on CC relaxation was not significantly inhibited by pretreatment with L-NAME, which is a NO synthesis inhibitor, or methylene blue, which is a guanylyl cyclase inhibitor. Therefore, we suggest that the CC relaxation effect was not related to the NO-cGMP pathway in acute alcohol administration.
The major nerve bundles involved in penile erections arise from the pelvic nerve, and the nerve bundles that inhibit penile erections originate from the sacral sympathetic nerve [
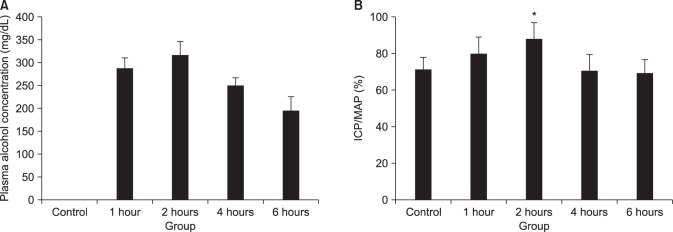
21]. Therefore, electrical stimulation of the pelvic nerve and the cavernosal nerve leads to erection. In our study, the blood alcohol concentration peaked 2 hours after alcohol administration
in vivo in rats, and the ICP/MAP percentage change due to the electric stimulation of the cavernosal nerve after acute alcohol administration was significantly higher in the 2-hour alcohol group than in the control group.
cAMP and cGMP are intracellular second messengers engaged in CC smooth muscle relaxation. They activate protein kinase A and protein kinase G, thereby activating K
+ channels and inactivating Ca
2+ channels, which prevents the intracellular infusion of Ca
2+, moves the intracellular Ca
2+ to the Golgi body, and causes relaxation of the CC smooth muscle [
22]. In our study, cAMP significantly increased in the CC of the 2-hour alcohol group, which showed the most erections.
Therefore, the CC relaxation observed after acute alcohol administration was caused by the activation of β-receptors, cyclooxygenase, and K+ channels, and the 2-hour alcohol group had a significantly greater ICP/MAP percentage and intra-cellular cAMP concentration. We therefore propose that acute alcohol administration causes erections by activating the cAMP pathway.
Lizarte et al [
23] performed an
in vitro organ bath study using rats divided into a chronic 4-week alcohol (20%) group and a 4-week water group, and reported that these 2 groups did not show significant differences in CC smooth muscle tissue contraction depending on the PE concentration. Our study similarly did not show a significant difference between the 12-week alcohol group and the control group. Therefore, our results confirm that chronic alcohol administration does not significantly affect hemodynamic responses to autonomic nerve stimulation.
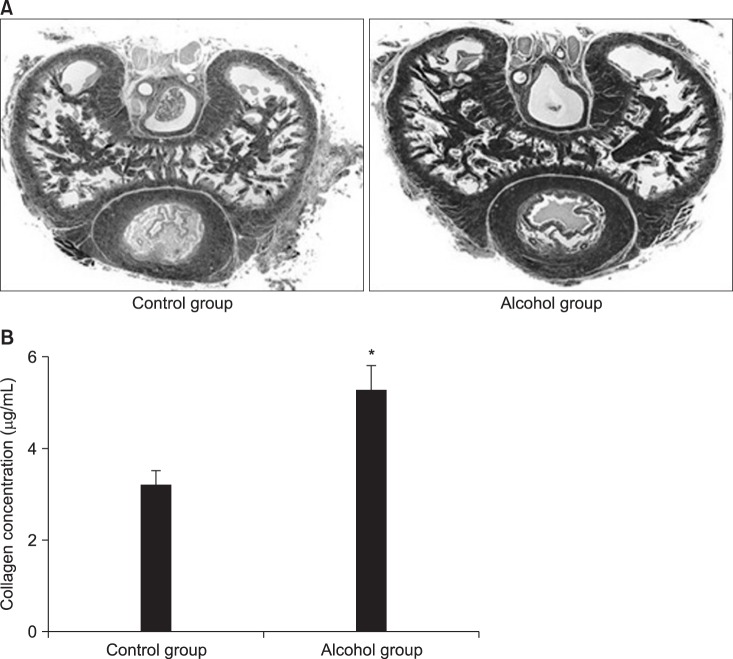
Elastic fibers, collagen fibers, and smooth muscle are known to play an important structural role in penile erections [
242526]. Our study found that smooth muscle in the CC significantly decreased, while collagen fibers increased and became denser in the 12-week alcohol group, and that the measured collagen level was significantly higher in the 12-week alcohol group. Therefore, chronic alcohol administration may cause ED as a result of histological ultrastructure changes in the CC.
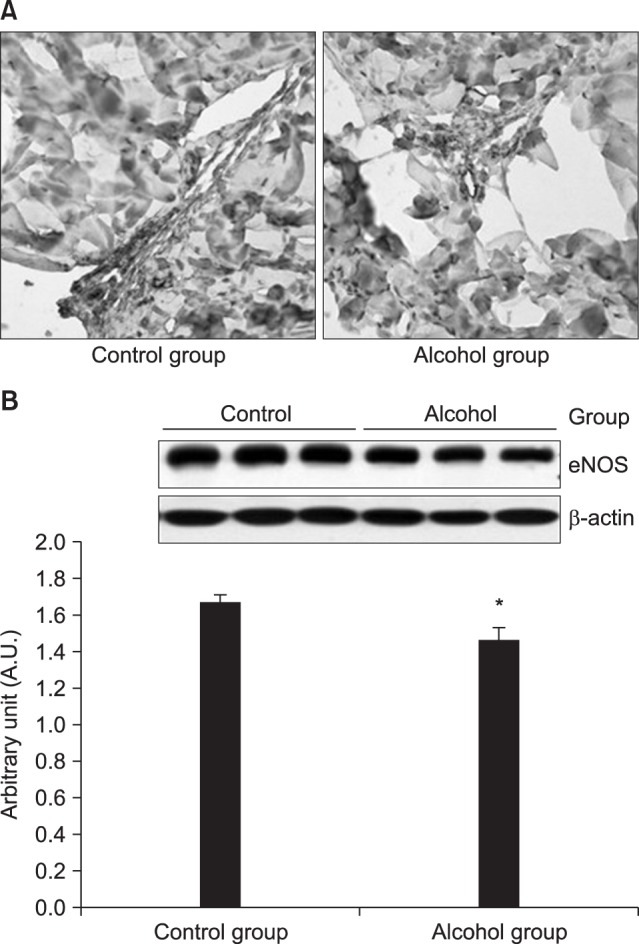
Our study found that that the eNOS expression regions were the same in both groups, but the response decreased in the 12-week alcohol group, and that eNOS expression was significantly lower in the 12-week alcohol group as determined by western blotting. Therefore, we suggest that chronic alcohol administration inhibits eNOS expression and can cause ED by reducing NO production.
Go to :





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download