Abstract
Purpose
The aim of this study was to assess the clinicopathologic characteristics of penile cancer, including patterns of therapy, oncologic results, and survival.
Materials and Methods
Between January 2005 and July 2015, 71 patients at 6 institutions who had undergone penectomy or penile biopsy were enrolled. Their medical records were reviewed to identify the mode of therapy, pathology reports, and cancer-specific survival (CSS) rate.
Results
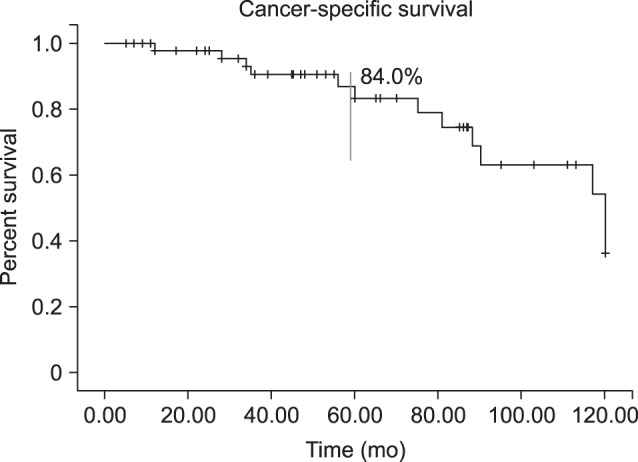
Clinicopathologic and outcome information was available for 52 male patients (mean age, 64.3 years; mean follow-up, 61.4 months). At presentation, 17 patients were node-positive, and 4 had metastatic disease. Management was partial penectomy in 34 patients, total penectomy in 12 patients, and chemotherapy or radiotherapy in 6 patients. The pathology reports were squamous cell carcinoma in 50 patients and other types of carcinoma in the remaining 2 patients. Kaplan-Meier survival analysis showed a 5-year CSS rate of 84.0%. In univariate and multivariate analyses, the American Joint Committee on Cancer (AJCC) stage and pathologic grade were associated with survival.
The incidence of primary penile cancer is very low in Western countries (<1.00 per 100,000 males) and is decreasing [1]. Primary penile cancer is also rare in Korea, with few cases reported. In Korea, penile cancer was diagnosed in 69 patients in 2011, corresponding to 0.06% of all malignancies in that year [2]. The incidence of penile cancer is related to general hygiene, cultural differences, and the prevalence of human papillomavirus (HPV) infection. HPV prevalence may account for the considerable geographic variation in the incidence of penile cancer [3].
Most penile cancer is squamous cell carcinoma and begins on the foreskin or the tip of the penis. Squamous cell carcinoma grows slowly; therefore, primary penile cancer can usually be cured if it is detected at an early stage. However, patients with carcinoma of the penis tend to delay seeking medical attention, with 65% delaying medical attention for more than 6 months after onset. This delay has been attributed to embarrassment, guilt, fear, ignorance, and personal neglect [4].
To assess the clinicopathologic characteristics of penile cancer, we present the first multicenter study of Korean patients, describing our experience with primary penile cancer patients treated at 6 tertiary academic hospitals.
We retrospectively reviewed the medical records of all cases of primary penile cancer treated at our institutions. Between January 2005 and July 2015, 71 such patients were identified at 6 institutions: Pusan National University Yangsan Hospital, Gyeongsang National University Hospital, Dong-A University Hospital, Kosin University Gospel Hospital, Inje University Haeundae Paik Hospital, and Pusan National University Hospital. The study protocol was exempt from the Institutional Review Board of Pusan National University Yangsan Hospital (IRB No. 05-2014-100).
The diagnosis was made according to clinical history, physical examination, and biopsy results. The primary treatment for penile lesions was partial or total penectomy, chemotherapy, and radiation therapy. Patients with clinically or radiologically evident inguinal or pelvic nodal involvement also underwent ipsilateral ilioinguinal lymphadenectomy with contralateral superficial inguinal or ilioinguinal dissection based on their clinical circumstances. Lymph node packets were dissected following standardized protocols. Tumor staging was standardized according to the American Joint Committee on Cancer (AJCC) system [5]. Tumors were graded as well, moderately, or poorly differentiated; carcinoma in situ was grouped with well-differentiated tumors for analysis.
Patients' records were reviewed to identify the mode of therapy, pathology reports, and cancer-specific survival (CSS) rate.
Categorical variables were compared using the chi-square test and the Fisher exact test. CSS rates were calculated by the Kaplan-Meier method as time from the date of surgery or biopsy to the date of progression or cancer-specific death; patients who were alive at the end of the study were censored at the last follow-up. Multivariate analysis was performed using a Cox proportional hazards model that included the AJCC disease stage and tumor grade as covariates. The p-values <0.05 were considered to indicate statistical significance. Statistical analyses were performed using PASW Statistics ver. 18.0 (IBM Co., Armonk, NY, USA).
A total of 71 cases met the inclusion criteria. Seventeen patients were excluded from the final analysis because of incomplete records or loss to follow-up. The clinicopathologic and outcome information of 52 male patients was available. The mean age at diagnosis was 64.3 years (range, 16~97 years). The mean follow-up was 61.4 months (range, 7~120 months). All patients were uncircumcised, with the exception of 2 cases of penile paraffinoma involving chronic inflammation of the penis. At presentation, 17 patients were node-positive, and 4 had metastatic disease. Initial treatment included partial penectomy (n=34, 65.4%), total penectomy (n=12, 23.1%) and chemotherapy or radiotherapy (n=6, 11.5%) (Table 1). Inguinal lymph node dissection was performed in 20 patients (43.5%). The most frequent pathologic diagnosis was squamous cell carcinoma (n=50, 96.2%) but leiomyosarcoma was observed in 2 cases (3.8%). The final specimens were staged as ≤pT1 (n=23, 44.2%), pT2 (n=13, 25.0%), pT3 (n=14, 26.9%), or pT4 (n=2, 3.8%). Overall, 35 patients (67.3%) had node-negative disease, 17 patients (32.7%) had node-positive disease, and 4 patients (7.7%) had metastatic disease. The penile cancer specimens showed largely mild to moderate differentiation (n=43, 82.7%) (Table 1). Kaplan-Meier survival analysis showed a 5-year CSS rate of 84.0% (Fig. 1).
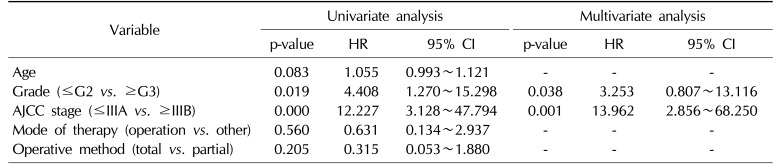
The associations of clinicopathologic variables with survival are shown in Table 2. Age, mode of therapy, and operative methods were not significantly associated with survival duration. However, the AJCC stage (p=0.000) and pathologic grade (p=0.019) were associated with survival (Fig. 2). In a multivariate Cox proportional hazards analysis, AJCC stage (p=0.001) and pathologic grade (p=0.038) were independent predictive factors for survival (Table 2).
Penile cancer is a rare solid cancer with an incidence of <1.0 per 100,000 males in Europe and the USA [1], but other parts of the world have a much higher incidence (1%~2% of male malignant disease) [6]. Countries and cultures practicing routine neonatal or youth circumcision have a lower incidence of penile cancer. The incidence of penile cancer is lowest in Israel (neonatal circumcision) and South Korea (youth circumcision) at 0.1 per 100,000 males; the majority of men in these countries are circumcised [27]. Penile cancer becomes more common with age and its incidence peaks during the sixth decade of life [8]. Most penile cancer patients in South Korea are elderly males in their 70s, 60s, and 50s, whose risk is elevated by 38.2%, 22.1%, and 20.6%, respectively [2].
The etiologic and epidemiologic risk factors for penile cancer are HPV infection, phimosis, smoking, and poor hygiene. HPV DNA is found in 70% to 100% of intraepithelial neoplasias, and up to 42% of penile cancer patients are HPV-positive [3]. The presence of phimosis is strongly associated with invasive penile cancer, likely due to smegma, which is associated with chronic infection and inflammation of the glans and prepuce [8]. Tobacco use, with a 4.5-fold increased risk (95% confidence interval, 2.0~10.1); chronic penile inflammation; and lichen sclerosus are also associated with an increased risk of penile cancer [910]. In contrast, circumcision helps to prevent retention of smegma, phimosis, and lichen sclerosus, and reduces the risk of HPV infection [11]. All patents in our study were uncircumcised, with the exception of 2 patients with penile paraffinoma.
Squamous cell carcinoma accounts for >95% of cases of malignant diseases of the penis. Penile squamous cell carcinoma has been described as a locoregional disease with a low incidence of distant metastasis. Moreover, most penile tumors are low-grade. Previous studies have suggested lymph node metastasis to be the main prognostic factor in this disease [121314]. In this study, 50 patients (96.2%) had squamous cell carcinoma and 43 patients (82.7%) had well to moderately differentiated tumors.
Lymphovascular or perineural invasion and histologic grade are strongly predictive of a poor prognosis and cancer-specific mortality [121314]. Any tumor with high-grade features is prone to having a poorer prognosis and a higher likelihood of metastasis [15]. We conducted a survival analysis using the Kaplan-Meier methodology and carried out univariate and multivariate analyses to identify prognostic factors. In the univariate analysis, AJCC stage and pathologic grade were significantly associated with a reduced survival rate. In a multivariate analysis using Cox regression, AJCC stage and pathologic grade were also associated with a reduced survival rate.
The presence of inguinal lymph node involvement was the most important prognostic factor in this study. Ravi [16] demonstrated a 5-year survival benefit (95% vs. 76%) in patients with no lymph node involvement (N0) versus inguinal lymph node involvement (N1 and N2). Our study showed a better prognosis in patients with AJCC stage less than IIIA than in those with stage IIIB and IV disease (Fig. 2A). Patients with cN0 disease who had a ~20% probability of micrometastases historically underwent superficial modified inguinal lymph node dissection for disease staging [17]. However, this procedure has complications in 10% to 36% of cases and is unnecessary in 80% of cases [1819]. Kroon et al [20] reported that patients with pT2 and pT3 tumors who underwent groin dissection had a 3-year CSS rate of 84%, compared to 35% in those who did not undergo groin lymph node dissection. However, in our study, we did not analyze this parameter because many urologists did not conduct inguinal lymph node dissection routinely, and only 20 patients underwent inguinal lymph node dissection.
The treatment of primary invasive malignancy of the penis has traditionally involved radical amputation with a 2-cm margin for oncologic efficacy. Although these procedures have been found to provide excellent local control, amputation is associated with considerable voiding dysfunction, as well as psychological and sexual morbidity [21]. The surgical treatment of primary invasive penile cancer has changed over the past 10 years from radical amputation to penile-sparing surgery. The most common procedure for PSS is glansectomy, which was first described in 1996 [22]. The recurrence rates for glansectomy are less than 4%, with excellent oncologic outcomes [2324252627]. Philippou et al [28] demonstrated that the rates of local recurrence and 5-year CSS after penile-sparing surgery were 8.9% and 91.7%, respectively, during a mean follow-up of 26 months. Bayles and Sethia [29] supported these excellent oncologic outcomes, reporting rates of local recurrence and cancer-specific mortality of 4.8% and 10.7%, respectively. In this study, the operation method (partial vs. total) was not significant for CSS.
This study had some limitations. First, it was an uncontrolled, retrospective study. All urologists who participated in this study did not use the same operative methods or decision-making strategies. Second, the number of patients in this study was small, and no central pathology review took place; therefore, further evaluation is necessary with a larger number of patients.
Primary penile cancer is a rare malignancy. In our study, partial penectomy was the most common treatment. The overall oncologic outcomes were good, with a 5-year CSS rate of 84.0%. The AJCC stage and pathologic grade were found to affect survival in a multivariate analysis. These findings provide insights into the cultural, social, and health-related behaviors related to penile cancer in Korea.
ACKNOWLEDGEMENTS
This study was supported by a research grant from Pusan National University Yangsan Hospital.
References
1. Chaux A, Netto GJ, Rodríguez IM, Barreto JE, Oertell J, Ocampos S, et al. Epidemiologic profile, sexual history, pathologic features, and human papillomavirus status of 103 patients with penile carcinoma. World J Urol. 2013; 31:861–867. PMID: 22116602.

2. Korean Ministry of Health and Welfare & Korean National Cancer Information Center 2015. Cancer in Korea [Internet]. Goyang: National Cancer Center;c2016. cited 2016 Jun 5. Available from: http://www.cancer.go.kr.
3. Bleeker MC, Heideman DA, Snijders PJ, Horenblas S, Dillner J, Meijer CJ. Penile cancer: epidemiology, pathogenesis and prevention. World J Urol. 2009; 27:141–150. PMID: 18607597.

4. Skeppner E, Andersson SO, Johansson JE, Windahl T. Initial symptoms and delay in patients with penile carcinoma. Scand J Urol Nephrol. 2012; 46:319–325. PMID: 22989150.

5. American Joint Committee on Cancer (AJCC). Penis. In : Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7th ed. New York: Springer;2010. p. 447–455.
6. Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB. Cancer incidence in five continents vol. viii. IARC Scientific Publication. Lyon: International Agency for Research on Cancer;2002. p. 93–514.
7. Morris BJ, Gray RH, Castellsague X, Bosch FX, Halperin DT, Waskett JH, et al. The strong protective effect of circumcision against cancer of the penis. Adv Urol. 2011; DOI: 10.1155/2011/812368.

8. Van Howe RS, Hodges FM. The carcinogenicity of smegma: debunking a myth. J Eur Acad Dermatol Venereol. 2006; 20:1046–1054. PMID: 16987256.

9. Daling JR, Madeleine MM, Johnson LG, Schwartz SM, Shera KA, Wurscher MA, et al. Penile cancer: importance of circumcision, human papillomavirus and smoking in in situ and invasive disease. Int J Cancer. 2005; 116:606–616. PMID: 15825185.
10. Archier E, Devaux S, Castela E, Gallini A, Aubin F, Le Maître M, et al. Carcinogenic risks of psoralen UV-A therapy and narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012; 26(Suppl 3):22–31. PMID: 22512677.

11. Lau WD, Ong CH, Lim TP, Teo C. Penile cancer: a local case series and literature review. Singapore Med J. 2015; 56:637–640. PMID: 26668410.
12. Cubilla AL. The role of pathologic prognostic factors in squamous cell carcinoma of the penis. World J Urol. 2009; 27:169–177. PMID: 18766352.

13. Lopes A, Hidalgo GS, Kowalski LP, Torloni H, Rossi BM, Fonseca FP. Prognostic factors in carcinoma of the penis: multivariate analysis of 145 patients treated with amputation and lymphadenectomy. J Urol. 1996; 156:1637–1642. PMID: 8863559.

14. Ornellas AA, Kinchin EW, Nóbrega BL, Wisnescky A, Koifman N, Quirino R. Surgical treatment of invasive squamous cell carcinoma of the penis: Brazilian National Cancer Institute long-term experience. J Surg Oncol. 2008; 97:487–495. PMID: 18425779.

15. Diorio GJ, Leone AR, Spiess PE. Management of penile cancer. Urology. 2016; 96:15–21. PMID: 26802797.

16. Ravi R. Correlation between the extent of nodal involvement and survival following groin dissection for carcinoma of the penis. Br J Urol. 1993; 72:817–819. PMID: 8281416.

17. Graafland NM, Lam W, Leijte JA, Yap T, Gallee MP, Corbishley C, et al. Prognostic factors for occult inguinal lymph node involvement in penile carcinoma and assessment of the high-risk EAU subgroup: a two-institution analysis of 342 clinically node-negative patients. Eur Urol. 2010; 58:742–747. PMID: 20800339.

18. Coblentz TR, Theodorescu D. Morbidity of modified prophylactic inguinal lymphadenectomy for squamous cell carcinoma of the penis. J Urol. 2002; 168:1386–1389. PMID: 12352399.

19. Bouchot O, Rigaud J, Maillet F, Hetet JF, Karam G. Morbidity of inguinal lymphadenectomy for invasive penile carcinoma. Eur Urol. 2004; 45:761–765. PMID: 15149749.

20. Kroon BK, Horenblas S, Lont AP, Tanis PJ, Gallee MP, Nieweg OE. Patients with penile carcinoma benefit from immediate resection of clinically occult lymph node metastases. J Urol. 2005; 173:816–819. PMID: 15711276.

21. Mikhail GR. Cancers, precancers, and pseudocancers on the male genitalia. A review of clinical appearances, histopathology, and management. J Dermatol Surg Oncol. 1980; 6:1027–1035. PMID: 6259230.

22. Austoni E, Fenice O, Kartalas Goumas Y, Colombo F, Mantovani F, Pisani E. New trends in the surgical treatment of penile carcinoma. Arch Ital Urol Androl. 1996; 68:163–168.
23. Gulino G, Sasso F, Falabella R, Bassi PF. Distal urethral reconstruction of the glans for penile carcinoma: results of a novel technique at 1-year of followup. J Urol. 2007; 178:941–944. PMID: 17632177.

24. O'Kane HF, Pahuja A, Ho KJ, Thwaini A, Nambirajan T, Keane P. Outcome of glansectomy and skin grafting in the management of penile cancer. Adv Urol. 2011; DOI: 10.1155/2011/240824.
25. Morelli G, Pagni R, Mariani C, Campo G, Menchini-Fabris F, Minervini R, et al. Glansectomy with split-thickness skin graft for the treatment of penile carcinoma. Int J Impot Res. 2009; 21:311–314. PMID: 19458620.

26. Palminteri E, Berdondini E, Lazzeri M, Mirri F, Barbagli G. Resurfacing and reconstruction of the glans penis. Eur Urol. 2007; 52:893–898. PMID: 17275169.

27. Smith Y, Hadway P, Biedrzycki O, Perry MJ, Corbishley C, Watkin NA. Reconstructive surgery for invasive squamous carcinoma of the glans penis. Eur Urol. 2007; 52:1179–1185. PMID: 17349734.

28. Philippou P, Shabbir M, Malone P, Nigam R, Muneer A, Ralph DJ, et al. Conservative surgery for squamous cell carcinoma of the penis: resection margins and long-term oncological control. J Urol. 2012; 188:803–808. PMID: 22818137.

29. Bayles AC, Sethia KK. The impact of improving outcomes guidance on the management and outcomes of patients with carcinoma of the penis. Ann R Coll Surg Engl. 2010; 92:44–45. PMID: 20056060.

Fig. 2
Kaplan-Meier estimated survival rate according to American Joint Committee on Cancer (AJCC) stage (A) and pathologic grade (B).
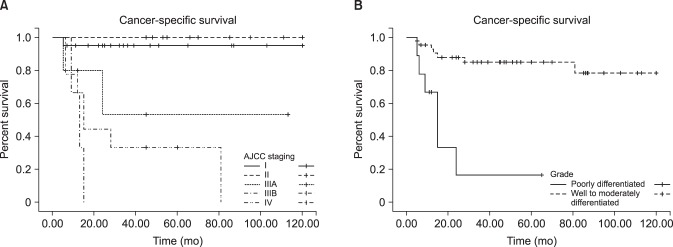
Table 1
Patient characteristics
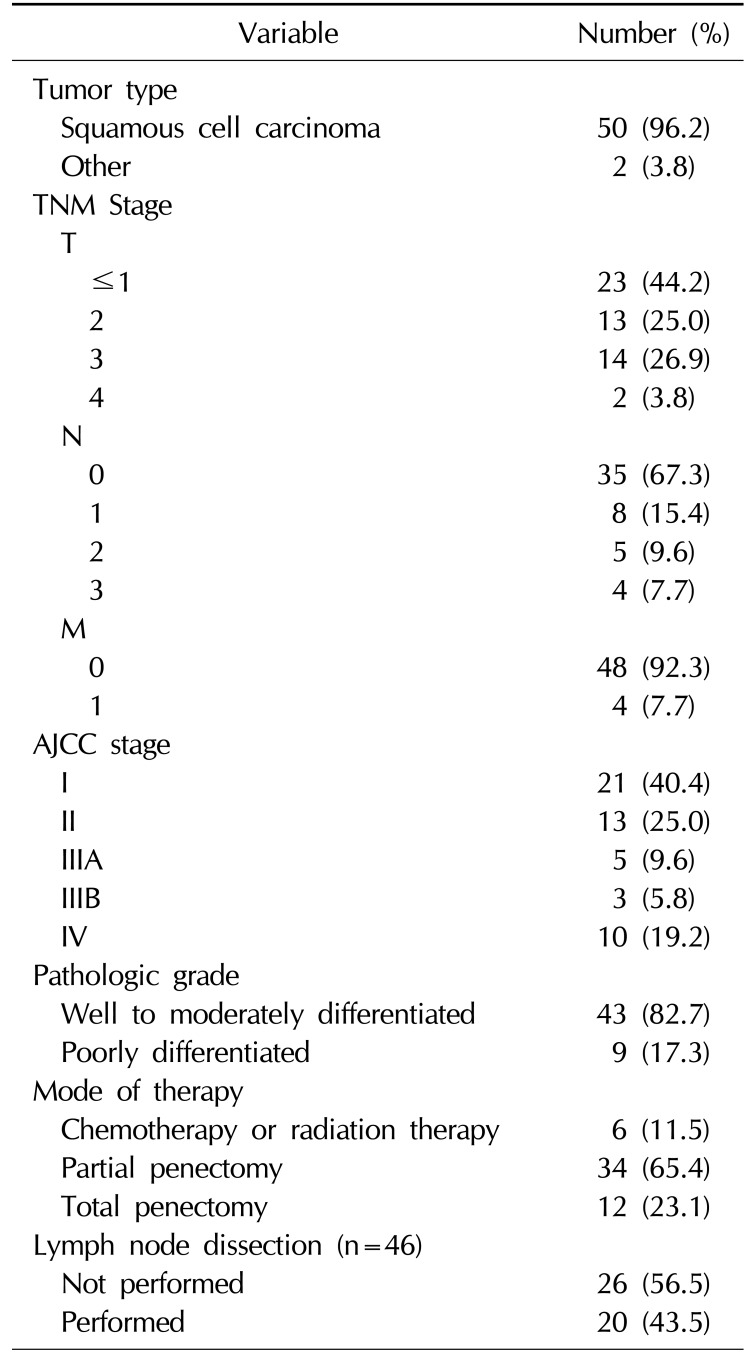
Table 2
Multivariable associations of clinicopathologic factors with survival




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download