Abstract
Purpose
Materials and Methods
Results
Conclusions
Figures and Tables
Fig. 1
Extracorporeal shockwave therapy (MT2000H) application to the rat penis. Under anesthesia, the prepuce was degloved and sutured to fix it.

Fig. 2
Intracavernosal pressure (ICP), ICP/mean arterial pressure (MAP), and area under the curve (AUC)/MAP measurements of each group. (A) ICP recordings after cavernous nerve stimulation. (B) Comparison of ICP/MAP ratios of the different groups. (C) Comparison of AUC/MAP ratios. Data are expressed as mean±standard deviation. aStatistical significance in comparison with the diabetes mellitus (DM)+extracorporeal shockwave therapy (ESWT) group. bStatistical significance in comparison with the DM group.

Fig. 3
Smooth muscle content evaluation of each group by Masson's trichrome staining (×100). The graph shows comparison of the smooth muscle and collagen fiber ratios of the different groups. Data are expressed as mean±standard deviation. aStatistical significance in comparison with the diabetes mellitus (DM)+extracorporeal shockwave therapy (ESWT) group. bStatistical significance in comparison with the DM group.
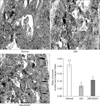
Fig. 4
(A) Immunohistochemical staining (×200) of vascular endothelial growth factor (VEGF) expression in the corpus cavernosum of each group. The graph shows comparison of VEGF expression in the different groups. (B) Immunohistochemical staining (×400) of neuronal nitric oxide synthase (nNOS) expression in the dorsal penile nerve of each group. The graph shows a comparison of nNOS expression levels of the different groups. Data are expressed as mean±standard deviation. aStatistical significance in comparison with the diabetes mellitus (DM)+extracorporeal shockwave therapy (ESWT) group. bStatistical significance in comparison with the DM group.

Fig. 5
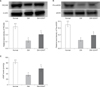
(A) Western blot images of platelet endothelial cell adhesion molecule-1 (PECAM-1).The graph shows a comparison of the relative PECAM-1/β-actin band densities of the different groups. (B) Pho-endothelial nitric oxide synthase (eNOS) expression in the corporal tissue of each group assessed by Western blotting. The graph shows a comparison of the relative band densities of Pho-eNOS/eNOS. (C) Cyclic guanosine monophosphate (cGMP) levels in the corporal tissue of each group as assessed by ELISA. Data are expressed as mean±standard deviation. aStatistical significance in comparison with the diabetes mellitus (DM)+extracorporeal shockwave therapy (ESWT) group. bStatistical significance in comparison with the DM group.
ACKNOWLEDGEMENTS
Notes
Author Contribution Research conception & design: Jeong HC, Kim KS, Kim SW. Performing the experiments: Jeon SH, Zhou GQ. Data acquisition: Bashraheel F, Choi SW. Data analysis and interpretation: Jeong HC, Kim SJ. Statistical analysis: Bae WJ, Cho HJ. Drafting of the manuscript: Jeong HC. Critical revision of the manuscript: Ha US, Hong SH, Lee JY, Moon DG. Receiving grant: Kim SW. Approval of final manuscript: all authors.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download