Abstract
Nocturia causes lack of sleep and excessive daytime somnolence, reducing overall well-being, vitality, productivity, and mental health. Nocturia is significantly associated with testosterone deficiency, lower urinary tract symptoms (LUTS), and sleep disorders. The development of LUTS is commonly associated with testosterone deficiency in elderly men, and recent studies have suggested that testosterone has an ameliorative effect on nocturia. In hypogonadal men with nocturia, a negative feedback cycle can arise, in which testosterone deficiency leads to the development of nocturia, and nocturia contributes to the decline in testosterone levels. Therefore, patients with nocturia should receive appropriate treatment in order to improve their quality of life. Nocturia is generally treated by restricting nighttime water intake, as well as by the administration of medications, such as alpha-1 blockers, anticholinergic drugs, and desmopressin. Testosterone replacement therapy (TRT) is used worldwide as a treatment for many hypogonadal conditions. TRT represents an alternative treatment option for nocturia in hypogonadal men. However, limited information is currently available regarding the effects of TRT on nocturia in hypogonadal men, and further studies are required to reach more definitive conclusions.
According to the International Continence Society criteria [1], nocturia is defined as the need to wake at night to void urine. Most people with severe nocturia, defined as waking 2 or more times per night to urinate, seek clinical treatment for the condition because it is associated with a reduction in health status and quality of life (QOL) [2345]. Waking at night to void causes lack of sleep and excessive daytime somnolence, reducing overall well-being, vitality, productivity, and mental health [23]. In particular, waking up to urinate 2 or more times per night is correlated with an increased risk of falling and limb/hip fractures [4]. Nocturia leads to increased mortality among elderly men, even after adjustment for possible contributory comorbidities and lifestyle factors [5]. Therefore, nocturia is a clinically significant condition.
Generally, nocturia becomes more common with age, and it is a characteristic clinical symptom of aging in the elderly [67]. Nocturia has a complex pathophysiology, and no direct cause has yet been identified. Various risk factors, such as aging, metabolic syndrome, hypertension, diabetes mellitus, congestive heart failure, sleep disorder, increased nocturnal urine volume, decreased bladder capacity, and benign prostate hypertrophy, have been suggested [8910]. In men, lower urinary tract symptoms (LUTS) occur concomitantly with an increase in prostate volume after 50 years of age. The incidence of LUTS increases with aging, with an overall prevalence of >50% in men aged >50 years, which exceeds the prevalence observed in elderly women [67].
However, an alternative causative factor is closely associated with nocturia in elderly men. Serum testosterone levels gradually decrease in elderly men, and their reduction is associated with the symptoms of late-onset hypogonadism (LOH) syndrome [11]. LOH syndrome comprises a cluster of clinical symptoms, including decreased libido, muscle weakness, increased visceral fat, obesity, osteoporosis, anemia, and reduced insulin sensitivity [11121314]. Recent studies have demonstrated that testosterone deficiency is also linked with the development of LUTS [15], and that testosterone replacement therapy (TRT) can improve LUTS in hypogonadal men with benign prostatic hyperplasia (BPH) [1617]. In addition, testosterone levels are potentially correlated with circadian rhythms and sleep quality, which are perturbed by nocturia. Indeed, several studies have reported that testosterone deficiency has a negative effect on overall sleep quality that can be attenuated by TRT [181920]. These findings suggest that testosterone has direct and indirect correlations with the development of nocturia in elderly men (Fig. 1).
However, little is known about the correlation between testosterone and nocturia. Furthermore, there is a lack of compelling data on the efficacy of TRT for the management of nocturia in hypogonadal men. Therefore, in this article, we review the relationship between testosterone and nocturia, and the possible effects of TRT on nocturia in hypogonadal men.
Many reports have investigated the relationship between testosterone and LUTS, whereas few have focused on the relationship between testosterone and nocturia in men [21222324252627]. Recent studies have suggested that testosterone has an ameliorative effect on nocturia (Table 1). Some of the benefits of TRT for nocturia can be explained by the effects of testosterone on sleep quality, urine-concentrating ability, metabolic syndrome, and LUTS/overactive bladder (OAB) (Fig. 2).
A cross-sectional study including 509 men aged 40 to 79 years found that serum testosterone levels were significantly associated with nocturia, and that men with serum testosterone levels in the upper quartile had a 44% lower risk of nocturia than men in the lowest quartile [21]. Another study involving 2,180 patients demonstrated that testosterone decreased 0.142 ng/mL for every time the patients woke at night to urinate, and that nocturia, especially nocturnal polyuria, was associated with decreased serum testosterone [22]. Kim et al [23] suggested that serum total testosterone levels may have a beneficial effect on lower urinary tract function, and showed that they were significantly lower in patients with 4 or more episodes of nocturia per night. In a cohort of 632 patients, patients with mean total testosterone levels of 2.21±0.51 ng/mL had a higher prevalence of nocturia [24]. Nocturia was also frequently observed among patients with lower testosterone levels who underwent transurethral prostate resection [25].
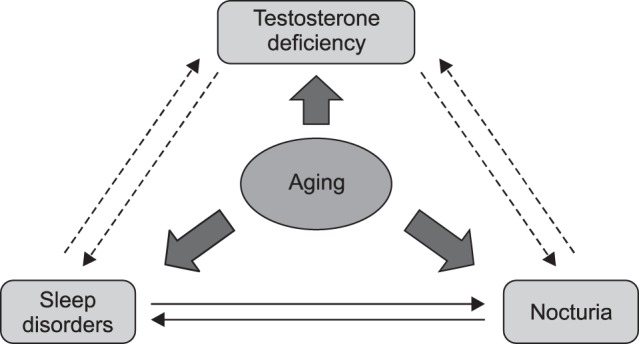
On the other hand, serum testosterone levels decrease significantly in men with nocturia, and low testosterone levels can be improved after 12 weeks of treatment with desmopressin [26]. Nocturia itself causes lack of sleep and decreases overall well-being, vitality, and mental health [23], which may contribute to a decrease in testosterone levels. Therefore, a negative feedback cycle can arise in hypogonadal men with nocturia, in which testosterone deficiency leads to the development of nocturia, and nocturia contributes to a decline in testosterone.
In contrast, another study suggested that men with higher serum testosterone levels may be at an increased risk of nocturia [27]. In 596 patients without BPH who were taking no medication for LUTS, nocturia showed a significant positive correlation with testosterone levels (odds ratio [OR]=1.15), suggesting that increased testosterone may contribute to the development of nocturia. However, the study population consisted of individuals who had relatively high testosterone levels (5.15±1.7 ng/mL) and were relatively young (47.1±7.4 years).
These conflicting data can be explained by differences among study populations and in the definitions of nocturia and LUTS, as well as by the influence of medications such as diuretics, anti-diabetic drugs, and alpha-1 blockers. Further studies are required for a more accurate understanding of the correlation between testosterone and nocturia.
Sleep disorders are an established cause of nocturia [28], and testosterone is significantly associated with sleep quality and sleep disorders. Some categories of the Aging Males' Symptoms Scale, which is commonly used to screen for specific symptoms of LOH syndrome, include questions regarding sleep quality. Recent studies have demonstrated that endogenous testosterone production is dependent on the first 3 hours of uninterrupted deep sleep, and that various sleep disorders, including abnormal sleep quality and duration, circadian rhythm disruption, sleep apnea syndrome (SAS), and nocturia, can reduce testosterone levels [181929].
Testosterone deficiency can also affect sleep [30313233]. A cohort study involving 1,312 men aged 65 years and older demonstrated that those with lower testosterone levels had lower sleep efficiency, with more frequent nocturnal awakenings and less non-rapid eye movement (REM) sleep [30]. Another study found that healthy young men with high endogenous testosterone levels experienced greater subjective sleepiness and reduced cognitive function after 5 days of sleep restriction than those with low testosterone levels [18]. In mice, the lack of testosterone following gonadectomy reduces the quality and quantity of non-REM sleep, but this effect can be attenuated by TRT [31]. A recent randomized study found that 6 months of TRT could improve sleep conditions, QOL, and nocturia in hypogonadal men with a clinical diagnosis of nocturia [32]. Testosterone is likely to play a role in sleep. Low testosterone may affect overall sleep quality, but this may be counteracted by TRT. Improved sleep quality as a result of TRT may have a favorable effect on nocturia in hypogonadal men with nocturia.
However, supratherapeutic doses of exogenous testosterone, also known as testosterone abuse, are reportedly associated with reduced sleep duration, insomnia, and nocturnal awakenings [1833]. Furthermore, SAS is known to have a negative effect on sleep quality and testosterone levels. Although TRT is not recommended for hypogonadal men with untreated SAS, there is a lack of evidence regarding the adverse effects of exogenous testosterone on SAS [34].
Testosterone is likely to be involved in the urine-concentrating ability of the kidneys. Nocturnal polyuria is considered the most significant contributory factor to nocturia. Indeed, a cross-sectional study including 2,180 patients with nocturia found that nocturnal polyuria was significantly associated with lower testosterone levels, and that individuals with low testosterone levels had greater nocturnal urine volume [22].
Several studies have demonstrated that testosterone plays a physiologic role in the maintenance of vasopressin levels and the urine-concentrating ability of the kidneys [35363738]. Experimental data have shown that, after orchiectomy, male rats exhibited decreased serum D-arginine-vasopressin and V2 vasopressin receptor expression, which reverted to normal with TRT [35]. The decreased number of vasopressin-binding sites in the kidneys of aging male rats has been found to be restored by TRT [36]. In addition, vasopressin is regulated by gonadal steroid hormones in the brain, with gonadectomy abolishing and TRT restoring normal vasopressin expression in adult men [37]. Furthermore, a clinical study investigating vasopressin levels via hypertonic saline infusions in hypogonadal men found that TRT improved the subnormal vasopressin response in aging men [38].
These studies suggest that loss of testosterone contributes to the development of nocturnal polyuria. However, little clinical evidence on the link between testosterone and polyuria is currently available, and further studies are required to reach a more definite conclusion.
Metabolic factors, such as obesity, hypertension, and insulin resistance, are associated with testosterone deficiency [121314], and several of these factors are also associated with nocturia [8910]. After 11 years of follow-up, a long-term longitudinal study determined that men with lower total testosterone levels are at an increased risk of developing metabolic syndrome, with an OR of 2.3 (95% confidence interval, 1.5~3.4) [39]. In addition, recent studies have found TRT to be effective in improving various metabolic factors [121314].
In long-term studies, testosterone deficiency has been found to be associated with the development of various metabolic parameters, which may become causes of nocturia in elderly men with hypogonadism. Over the long term, TRT may improve nocturia indirectly by ameliorating various metabolic factors.
Nocturia is prevalent among elderly men with BPH and LUTS, and has the most serious symptoms in elderly men. Recent studies have demonstrated that testosterone plays an important role in the development of LUTS and OAB in elderly men [15]. The reduced bladder capacity caused by BPH/LUTS and OAB in men is associated with nocturia.
Androgen receptors are found in the urothelium, including in the urinary bladder, prostate, and urethra. Testosterone modulates the autonomic nervous system and the activities of Rho-kinase, nitric oxide (NO) synthase, and phosphodiesterase-5 (PDE5) through these androgen receptors. Furthermore, testosterone activates endothelial NO synthase in the pelvis, consequently increasing NO concentration in the tissues of blood vessels, which may result in the dilation of pelvic vessels and alleviation of pelvic ischemia. Bladder blood flow is often decreased in patients with BPH and LUTS [40], and decreased bladder blood flow and ischemia caused by aging and arterial sclerosis are associated with the development of detrusor overactivity [4142]. Therefore, it may be possible to alter the interaction between testosterone and the urinary tract with NO, which may improve bladder neck relaxation, bladder capacity, and pelvic blood flow. Indeed, previous studies based on pressure-flow analysis and uroflowmetry have demonstrated that TRT for 1 year in hypogonadal men with LUTS effectively increased maximal bladder capacity and bladder compliance, and decreased detrusor pressure at maximal flow [1617].
Furthermore, recent clinical trials have reported that short-term TRT improved LUTS in hypogonadal men with BPH [324344454647]. However, limited information is available regarding the effects and safety of longer-term TRT in men with severe BPH and LUTS, and clinical reports on the effects of TRT on nocturia and OAB remain scarce. Further studies are required to reach more definitive conclusions.
As described earlier, some clinical reports have asserted that TRT can improve LUTS in hypogonadal men with BPH, whereas the results of a very few studies have suggested that TRT may have a positive effect on nocturia (Table 2).
Kalinchenko et al [44] reported that 2 types of testosterone administration (testosterone gel or intramuscular testosterone undecanoate injection) for 26 weeks improved nocturia, total International Prostate Symptom Score (IPSS), symptoms of irritation, and obstructive symptoms in 30 hypogonadal men. In another case series study, application of testosterone ointment for 3 months by 41 patients with LOH syndrome improved LUTS and nocturia, achieving an increase in serum testosterone levels [45]. Moreover, TRT for over 1 year in patients with moderate LUTS receiving no medication for BPH improved both storage and voiding symptoms [46]. A recent sub-analysis of a randomized clinical trial examining the effects of TRT on LOH syndrome found that 6 months of TRT improved nocturia, sleep conditions, and QOL in hypogonadal men with nocturia [32]. Yassin et al [47] reported that TRT improved the IPSS score, nocturia, residual voiding volume, and bladder wall thickness, and that TRT interruption led these clinical conditions to worsen. Based on a pressure-flow study, TRT was found to contribute to a significant increase in prostate volume and an improvement in the IPSS score, and significantly increased maximal bladder capacity and compliance, which may improve storage symptoms, including nocturia [16].
However, there are some important limitations of these studies, in which nocturia was evaluated based on the scores for question 7 of the IPSS. This question is solely based on patients' subjective symptoms, and does not take into account a voiding diary or nocturnal urine volume. In addition, the safety and effects of TRT on urinary symptoms in patients with severe BPH or post-void residual urine volume have not yet been established. It is unclear whether the use of an alpha-1 blocker or PDE-5 inhibitor combined with testosterone is recommended in these patients. Further studies including patients with severe BPH/LUTS and the assessment of urine-concentrating disorders at nighttime with a voiding diary are required to understand the clinical effects of TRT on nocturia.
To improve QOL in patients with nocturia, their symptoms are generally addressed by restricting nighttime water intake and by treatment with medications, such as alpha 1-blockers, anticholinergic drugs, desmopressin, and drugs to induce sleep. However, a recent report demonstrated that nocturia treatment only reduced nocturnal voiding frequency, without improving QOL [5].
Various studies have suggested significant associations among nocturia, testosterone deficiency, LUTS/OAB, and sleep disorders. A negative feedback cycle can arise in hypogonadal men with nocturia, in which testosterone deficiency leads to the development of nocturia, and nocturia contributes to a decline in testosterone levels. Therefore, we should treat patients with nocturia appropriately to improve their QOL.
TRT is used worldwide as a treatment for many hypogonadal conditions, such as the loss of muscle mass and strength, reduced bone mineral density, sexual dysfunction, deterioration of insulin resistance, elevated visceral fat, and metabolic syndrome. As an additional benefit, TRT may also improve nocturia in hypogonadal men. However, limited information is currently available regarding the effects of TRT on nocturia in hypogonadal men, and further studies, including long-term observations of patients with varying severities of BPH or LUTS, are required to reach more definitive conclusions.
References
1. van Kerrebroeck P, Abrams P, Chaikin D, Donovan J, Fonda D, Jackson S, et al. The standardisation of terminology in nocturia: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002; 21:179–183. PMID: 11857672.

2. Kobelt G, Borgström F, Mattiasson A. Productivity, vitality and utility in a group of healthy professionally active individuals with nocturia. BJU Int. 2003; 91:190–195. PMID: 12581002.

3. Asplund R, Marnetoft SU, Selander J, Akerström B. Nocturia in relation to somatic health, mental health and pain in adult men and women. BJU Int. 2005; 95:816–819. PMID: 15794789.

4. Nakagawa H, Niu K, Hozawa A, Ikeda Y, Kaiho Y, Ohmori-Matsuda K, et al. Impact of nocturia on bone fracture and mortality in older individuals: a Japanese longitudinal cohort study. J Urol. 2010; 184:1413–1418. PMID: 20727545.

5. Kim SO, Choi HS, Kim YJ, Kim HS, Hwang IS, Hwang EC, et al. Impact of nocturia on health-related quality of life and medical outcomes study sleep score in men. Int Neurourol J. 2011; 15:82–86. PMID: 21811697.

6. Homma Y, Yamaguchi O, Hayashi K. Neurogenic Bladder Society Committee. Epidemiologic survey of lower urinary tract symptoms in Japan. Urology. 2006; 68:560–564. PMID: 16979726.

7. Tikkinen KA, Tammela TL, Huhtala H, Auvinen A. Is nocturia equally common among men and women? A population based study in Finland. J Urol. 2006; 175:596–600. PMID: 16407003.

8. Asplund R. Nocturia in relation to sleep, somatic diseases and medical treatment in the elderly. BJU Int. 2002; 90:533–536. PMID: 12230611.

9. Fitzgerald MP, Litman HJ, Link CL, McKinlay JB. BACH Survey Investigators. The association of nocturia with cardiac disease, diabetes, body mass index, age and diuretic use: results from the BACH survey. J Urol. 2007; 177:1385–1389. PMID: 17382738.

10. Yoshimura K, Terada N, Matsui Y, Terai A, Kinukawa N, Arai Y. Prevalence of and risk factors for nocturia: analysis of a health screening program. Int J Urol. 2004; 11:282–287. PMID: 15147543.

11. Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Baltimore Longitudinal Study of Aging. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab. 2001; 86:724–731. PMID: 11158037.

12. Lunenfeld B, Mskhalaya G, Zitzmann M, Arver S, Kalinchenko S, Tishova Y, et al. Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men. Aging Male. 2015; 18:5–15. PMID: 25657080.

13. Bassil N, Alkaade S, Morley JE. The benefits and risks of testosterone replacement therapy: a review. Ther Clin Risk Manag. 2009; 5:427–448. PMID: 19707253.
14. Huhtaniemi I. Late-onset hypogonadism: current concepts and controversies of pathogenesis, diagnosis and treatment. Asian J Androl. 2014; 16:192–202. PMID: 24407185.

15. Shigehara K, Namiki M. Late-onset hypogonadism syndrome and lower urinary tract symptoms. Korean J Urol. 2011; 52:657–663. PMID: 22087358.

16. Karazindiyanoğlu S, Cayan S. The effect of testosterone therapy on lower urinary tract symptoms/bladder and sexual functions in men with symptomatic late-onset hypogonadism. Aging Male. 2008; 11:146–149. PMID: 18821291.
17. Shigehara K, Sugimoto K, Konaka H, Iijima M, Fukushima M, Maeda Y, et al. Androgen replacement therapy contributes to improving lower urinary tract symptoms in patients with hypogonadism and benign prostate hypertrophy: a randomised controlled study. Aging Male. 2011; 14:53–58. PMID: 21171937.

18. Wittert G. The relationship between sleep disorders and testosterone in men. Asian J Androl. 2014; 16:262–265. PMID: 24435056.

19. Schmid SM, Hallschmid M, Jauch-Chara K, Lehnert H, Schultes B. Sleep timing may modulate the effect of sleep loss on testosterone. Clin Endocrinol (Oxf). 2012; 77:749–754. PMID: 22568763.

20. Oh MM, Kim JW, Jin MH, Kim JJ, Moon du G. Influence of paradoxical sleep deprivation and sleep recovery on testosterone level in rats of different ages. Asian J Androl. 2012; 14:330–334. PMID: 22157981.

21. Liao CH, Chiang HS, Yu HJ. Serum testosterone levels significantly correlate with nocturia in men aged 40-79 years. Urology. 2011; 78:631–635. PMID: 21782223.

22. Kim JW, Oh MM, Yoon CY, Bae JH, Kim JJ, Moon du G. Nocturnal polyuria and decreased serum testosterone: is there an association in men with lower urinary tract symptoms? Int J Urol. 2014; 21:518–523. PMID: 24286364.

23. Kim MK, Zhao C, Kim SD, Kim DG, Park JK. Relationship of sex hormones and nocturia in lower urinary tract symptoms induced by benign prostatic hyperplasia. Aging Male. 2012; 15:90–95. PMID: 22385128.

24. Liu HY, Chung MS, Wang HJ, Liu RT, Chuang YC. Nocturia indicates a poor health status and increases mortality in male patients with type 2 diabetes mellitus. Int Urol Nephrol. 2016; 48:1209–1214. PMID: 27156073.

25. Wu Y, Pan H, Wang WM, Xu D, Zhang L, Gu ZQ, et al. A possible relationship between serum sex hormones and benign prostatic hyperplasia/lower urinary tract symptoms in men who underwent transurethral prostate resection. Asian J Androl. 2017; 19:230–233. PMID: 26763548.

26. Kim JW, Chae JY, Kim JW, Yoon CY, Oh MM, Park HS, et al. Can treatment of nocturia increase testosterone level in men with late onset hypogonadism? Urology. 2014; 83:837–842. PMID: 24680454.

27. Jeh SU, Yoon S, Seo DH, Lee SW, Lee C, Choi SM, et al. Relationship between serum testosterone and nocturia in men without benign prostate enlargement. Andrology. 2017; 5:58–62. PMID: 27636882.

28. Yoo SS, Shim BS, Lee DH, Lee HW, Yoon H. Correlation between nocturia and sleep: a questionnaire based analysis. Korean J Urol. 2010; 51:757–762. PMID: 21165195.

29. Luboshitzky R, Zabari Z, Shen-Orr Z, Herer P, Lavie P. Disruption of the nocturnal testosterone rhythm by sleep fragmentation in normal men. J Clin Endocrinol Metab. 2001; 86:1134–1139. PMID: 11238497.

30. Barrett-Connor E, Dam TT, Stone K, Harrison SL, Redline S, Orwoll E. Osteoporotic Fractures in Men Study Group. The association of testosterone levels with overall sleep quality, sleep architecture, and sleep-disordered breathing. J Clin Endocrinol Metab. 2008; 93:2602–2609. PMID: 18413429.

31. Paul KN, Laposky AD, Turek FW. Reproductive hormone replacement alters sleep in mice. Neurosci Lett. 2009; 463:239–243. PMID: 19647784.

32. Shigehara K, Konaka H, Koh E, Izumi K, Kitagawa Y, Mizokami A, et al. Effects of testosterone replacement therapy on nocturia and quality of life in men with hypogonadism: a subanalysis of a previous prospective randomized controlled study in Japan. Aging Male. 2015; 18:169–174. PMID: 26075538.

33. Liu PY, Yee B, Wishart SM, Jimenez M, Jung DG, Grunstein RR, et al. The short-term effects of high-dose testosterone on sleep, breathing, and function in older men. J Clin Endocrinol Metab. 2003; 88:3605–3613. PMID: 12915643.

34. Hanafy HM. Testosterone therapy and obstructive sleep apnea: is there a real connection? J Sex Med. 2007; 4:1241–1246. PMID: 17645445.

35. Pávó I, Varga C, Szücs M, László F, Szécsi M, Gardi J, et al. Effects of testosterone on the rat renal medullary vasopressin receptor concentration and the antidiuretic response. Life Sci. 1995; 56:1215–1222. PMID: 7475899.

36. Herzberg NH, Goudsmit E, Kruisbrink J, Boer GJ. Testosterone treatment restores reduced vasopressin-binding sites in the kidney of the ageing rat. J Endocrinol. 1989; 123:59–63. PMID: 2809490.

37. Pak TR, Chung WC, Hinds LR, Handa RJ. Estrogen receptor-beta mediates dihydrotestosterone-induced stimulation of the arginine vasopressin promoter in neuronal cells. Endocrinology. 2007; 148:3371–3382. PMID: 17412808.
38. Ikeda Y, Tanaka I, Oki Y, Gemmma R, Morita H, Komatsu K, et al. Testosterone normalizes plasma vasopressin response to osmotic stimuli in men with hypogonadism. Endocr J. 1993; 40:387–392. PMID: 7920892.

39. Laaksonen DE, Niskanen L, Punnonen K, Nyyssönen K, Tuomainen TP, Valkonen VP, et al. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care. 2004; 27:1036–1041. PMID: 15111517.

40. Mitterberger M, Pallwein L, Gradl J, Frauscher F, Neuwirt H, Leunhartsberger N, et al. Persistent detrusor overactivity after transurethral resection of the prostate is associated with reduced perfusion of the urinary bladder. BJU Int. 2007; 99:831–835. PMID: 17244278.

41. McVary KT. Erectile dysfunction and lower urinary tract symptoms secondary to BPH. Eur Urol. 2005; 47:838–845. PMID: 15925081.

42. Azadzoi KM, Tarcan T, Kozlowski R, Krane RJ, Siroky MB. Overactivity and structural changes in the chronically ischemic bladder. J Urol. 1999; 162:1768–1778. PMID: 10524933.

43. Saad F, Gooren LJ, Haider A, Yassin A. A dose-response study of testosterone on sexual dysfunction and features of the metabolic syndrome using testosterone gel and parenteral testosterone undecanoate. J Androl. 2008; 29:102–105. PMID: 17916569.

44. Kalinchenko S, Vishnevskiy EL, Koval AN, Mskhalaya GJ, Saad F. Beneficial effects of testosterone administration on symptoms of the lower urinary tract in men with late-onset hypogonadism: a pilot study. Aging Male. 2008; 11:57–61. PMID: 18570056.

45. Amano T, Imao T, Takemae K, Iwamoto T, Nakanome M. Testosterone replacement therapy by testosterone ointment relieves lower urinary tract symptoms in late onset hypogonadism patients. Aging Male. 2010; 13:242–246. PMID: 20795793.

46. Ko YH, Moon du G, Moon KH. Testosterone replacement alone for testosterone deficiency syndrome improves moderate lower urinary tract symptoms: one year follow-up. World J Mens Health. 2013; 31:47–52. PMID: 23658865.

47. Yassin A, Nettleship JE, Talib RA, Almehmadi Y, Doros G. Effects of testosterone replacement therapy withdrawal and re-treatment in hypogonadal elderly men upon obesity, voiding function and prostate safety parameters. Aging Male. 2016; 19:64–69. PMID: 26742589.

Fig. 2
Possible mechanisms of the influence of testosterone deficiency on the development of nocturia. Testosterone has an ameliorative effect on nocturia. The benefits of testosterone replacement therapy can be explained by the effects of testosterone on sleep quality, urine-concentrating ability, metabolic syndrome, and lower urinary tract symptoms/overactive bladder. LUTS: lower urinary tract symptoms, OAB: overactive bladder.
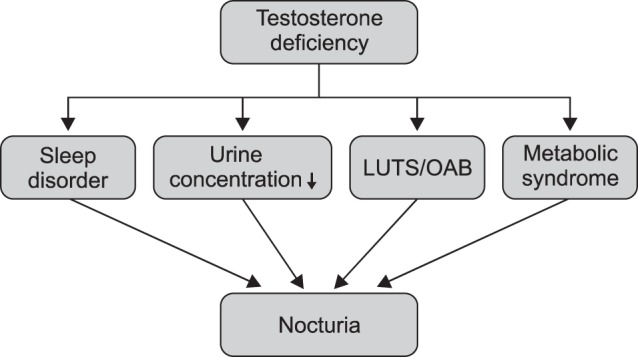
Table 1
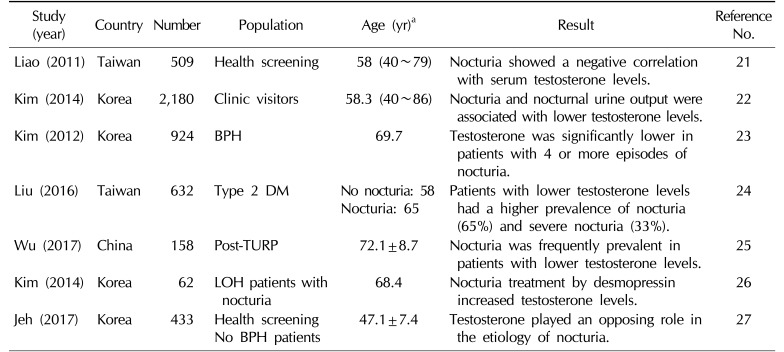
Relationships between testosterone levels and nocturia
| Study (year) | Country | Number | Population | Age (yr)a | Result | Reference No. |
|---|---|---|---|---|---|---|
| Liao (2011) | Taiwan | 509 | Health screening | 58 (40~79) | Nocturia showed a negative correlation with serum testosterone levels. | 21 |
| Kim (2014) | Korea | 2,180 | Clinic visitors | 58.3 (40~86) | Nocturia and nocturnal urine output were associated with lower testosterone levels. | 22 |
| Kim (2012) | Korea | 924 | BPH | 69.7 | Testosterone was significantly lower in patients with 4 or more episodes of nocturia. | 23 |
| Liu (2016) | Taiwan | 632 | Type 2 DM |
No nocturia: 58 Nocturia: 65 |
Patients with lower testosterone levels had a higher prevalence of nocturia (65%) and severe nocturia (33%). |
24 |
| Wu (2017) | China | 158 | Post-TURP | 72.1±8.7 | Nocturia was frequently prevalent in patients with lower testosterone levels. | 25 |
| Kim (2014) | Korea | 62 |
LOH patients with nocturia |
68.4 | Nocturia treatment by desmopressin increased testosterone levels. | 26 |
| Jeh (2017) | Korea | 433 | Health screening No BPH patients | 47.1±7.4 | Testosterone played an opposing role in the etiology of nocturia. | 27 |
Table 2
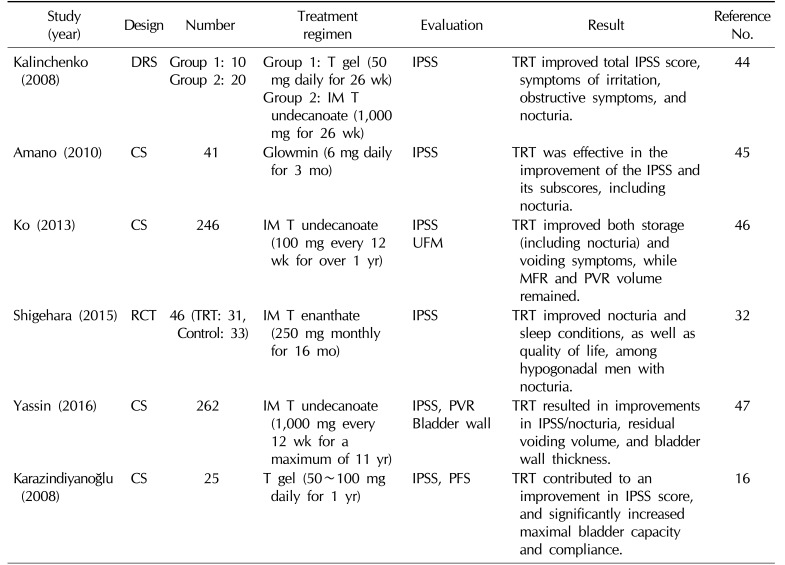
The effects of testosterone replacement therapy on nocturia
| Study (year) | Design | Number | Treatment regimen | Evaluation | Result | Reference No. |
|---|---|---|---|---|---|---|
| Kalinchenko (2008) | DRS |
Group 1: 10 Group 2: 20 |
Group 1: T gel (50 mg daily for 26 wk) Group 2: IM T undecanoate (1,000 mg for 26 wk) |
IPSS | TRT improved total IPSS score, symptoms of irritation, obstructive symptoms, and nocturia. | 44 |
| Amano (2010) | CS | 41 | Glowmin (6 mg daily for 3 mo) | IPSS | TRT was effective in the improvement of the IPSS and its subscores, including nocturia. | 45 |
| Ko (2013) | CS | 246 | IM T undecanoate (100 mg every 12 wk for over 1 yr) | IPSS UFM | TRT improved both storage (including nocturia) and voiding symptoms, while MFR and PVR volume remained. | 46 |
| Shigehara (2015) | RCT | 46 (TRT: 31, Control: 33) | IM T enanthate (250 mg monthly for 16 mo) | IPSS | TRT improved nocturia and sleep conditions, as well as quality of life, among hypogonadal men with nocturia. | 32 |
| Yassin (2016) | CS | 262 | IM T undecanoate (1,000 mg every 12 wk for a maximum of 11 yr) |
IPSS, PVR Bladder wall |
TRT resulted in improvements in IPSS/nocturia, residual voiding volume, and bladder wall thickness. | 47 |
| Karazindiyanoğlu (2008) | CS | 25 | T gel (50~100 mg daily for 1 yr) | IPSS, PFS | TRT contributed to an improvement in IPSS score, and significantly increased maximal bladder capacity and compliance. | 16 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download