Abstract
Purpose
Much attention has been focused in recent decades on the effects of erectile dysfunction (ED) secondary to lower urinary tract symptoms (LUTS), potentially underestimating its effects in men without LUTS. This study aimed to compare the prevalence and predictors of ED in men with and without LUTS.
Materials and Methods
The International Index of Erectile Function questionnaire was administered to 303 patients between January 2014 and June 2016. Within this sample, 147 patients with LUTS (cases) were compared to 156 men without LUTS who were matched for age, level of education, and occupation (controls).
Results
The mean age was 66.03±9.64 years and 65.78±8.61 years for the cases and controls, respectively. The prevalence of ED was 64.6% and 73.7% (odds ratio [OR], 1.54; 95% confidence interval [CI], 0.94∼2.51) in the case cohort and controls, respectively (p=0.086). There was no difference in the prevalence of impaired erectile function (p=0.067), impaired orgasmic function (p=0.108), impaired sexual desire (p=0.291), impaired intercourse satisfaction (p=0.869), or impaired overall satisfaction (p=0.191). Multivariate logistic regression analysis showed that being currently employed was a significant predictor of ED both in men with LUTS (OR, 8.08; 95% CI, 1.51∼9.27; p=0.004) and in men without LUTS (OR, 7.00; 95% CI, 1.49∼14.51; p=0.008). Being married only predicted for impaired EF in men without LUTS (OR, 6.34; 95% CI, 1.40∼15.20; p<0.05).
The incidence of sexual problems in the general population has been high in recent decades, and erectile dysfunction (ED) has been reported to be one of the most common types of sexual dysfunction in men worldwide [12]. The estimated global prevalence of ED has steadily increased, and it is projected that the number of men with this condition will rise to 322 million by the year 2025 [3]. ED is usually underestimated in many developing countries, including Nigeria [456]. This is probably because it is not a life-threatening condition; moreover, due to the associated stigma, men with the problem rarely seek help. There is also the problem of early detection and management of the factors responsible for the development of ED. The prevalence of ED has been shown to vary from region to region within Nigeria depending on the method of evaluation [789].
The impact of ED can be devastating, as evidence has shown that sexual function is an important index of quality of life [91011]. ED can affect all levels of intimacy, including the emotional, social, sexual, recreational, and intellectual domains [11]. Previous studies have reported significantly impaired health-related quality of life in men with ED [12]. This has been found to affect both general and disease-specific health-related quality of life. Therefore, urgent attention is required to address this issue.
In recent decades, considerable attention has been focused on the correlation between lower urinary tract symptoms (LUTS) and ED, thereby underestimating the burden of ED in men without LUTS [111314]. Some descriptive studies have suggested that LUTS are a risk factor for ED, independent of age and other comorbidities [15]. The pattern of ED should also be determined in patients without LUTS. This will enable us to determine whether men without LUTS have a similar or higher prevalence of ED compared to men with LUTS.
This study was undertaken to determine the prevalence and predictors of ED in men with and without LUTS among patients receiving care at Ekiti State University Teaching Hospital, Southwestern Nigeria.
This was a cross-sectional study conducted at Ekiti State University Teaching Hospital, Ado-Ekiti among all new patients who presented from January 1, 2014 to June 30, 2016.
A total of 303 men were recruited during the study period. This included 147 patients who presented to the urology clinic with LUTS and 156 patients without LUTS attending the general outpatient clinic of the hospital.
Subjects who were 18 years old (our cut-off age for adult males) and above, who had been involved in sexual activity within 4 to 6 weeks of presentation, and who were willing to participate in the study as indicated by signing the consent form were included in the study. All recruitment was conducted within the study period of 2 and a half years.
The control group comprised subjects within a similar age range as the LUTS patients, who had engaged in sexual activity within 4 to 6 weeks prior to presentation, and who were willing to participate in the study as indicated by signing the consent form. All recruitment was conducted within the same study period as was used for men with LUTS.
Subjects who had not engaged in sexual activity within 4 to 6 weeks prior to presentation were excluded, as well as those who did not agree to participate in the study. Subjects <18 years of age, which was our cut-off year for adult males, or who had no sexual partner were also excluded.
Subjects who had not engaged in sexual activity within 4 to 6 weeks prior to presentation or were not willing to participate in the study were excluded. Subjects <18 years of age and those with a past or current diagnosis of LUTS were also excluded.
(1) A questionnaire was drawn up by the researchers to elicit information on the socio-demographic characteristics of respondents and their clinical characteristics.
(2) The International Index of Erectile Function (IIEF) questionnaire was used, and all items were scored in all 5 domains [16].
A total IIEF score of ≥60 was interpreted as indicating the absence of ED, while a total IIEF score of <60 was interpreted as signifying ED.
Ethical approval for the study was obtained from the Research and Ethics Committee of Ekiti State University Teaching Hospital, Ado-Ekiti, Nigeria (EKSUTH/A67/2014/01/006).
Trained physicians from the urology department interviewed each subject in an individual room, ensuring privacy and confidentiality.
IBM SPSS ver. 21.0 for Windows (IBM Co., Armonk, NY, USA) was used for data analysis. The data were summarized using frequencies and percentages. Continuous variables were summarized as the mean and standard deviation. Between-group differences were tested with the Student t-test for normally distributed numerical data, while the Pearson chi-square test was used to compare proportions. Multivariate binary logistic regression analysis was performed to determine the predictors of ED in both populations. The level of significance was p<0.05.
A total of 303 patients were interviewed. There were 147 cases and 156 controls, with a ratio of 1:1. The mean age of the cases was 66.03±9.64 years, while that of the controls was 65.78±8.61 years (p=0.806).
The mean body mass index was 26.92±3.98 kg/m2 and 25.47±3.88 kg/m2 for the case cohort and controls, respectively (p=0.002). The prevalence of ED was higher in the controls than in the cases, with values of 73.7% and 64.6%, respectively (p=0.086).
Table 1 show that the data in the two groups were well matched and therefore quite comparable. The prevalence of currently married men was higher in the case cohort (79.6%) than in the controls (52.6%; p<0.001).
More men smoked in the case cohort than in the controls (p=0.034). The same pattern was found for alcohol consumption and obesity/overweight (p=0.038).
In addition, the prevalence of comorbidities was higher in the controls than the case cohort (48.7% and 42.2%, respectively; p<0.001). A previous history of pelvic trauma was higher in the controls than in the case cohort (p<0.001). Most controls were self-employed, while retired men were most prevalent among the cases.
Table 2 shows that the mean scores for erectile function (EF), orgasmic function (OF), sexual desire (SD), intercourse satisfaction (IS), and overall satisfaction (OS) were non-significantly higher in the controls than in the case cohort.
Table 3 shows the prevalence of participants with impairments as indicated by the IIEF. It was revealed that the prevalence of IS was equal in the case cohort and the controls (p=0.869), but the prevalence of EF, OF, SD, and OS was higher in the controls (p>0.05).
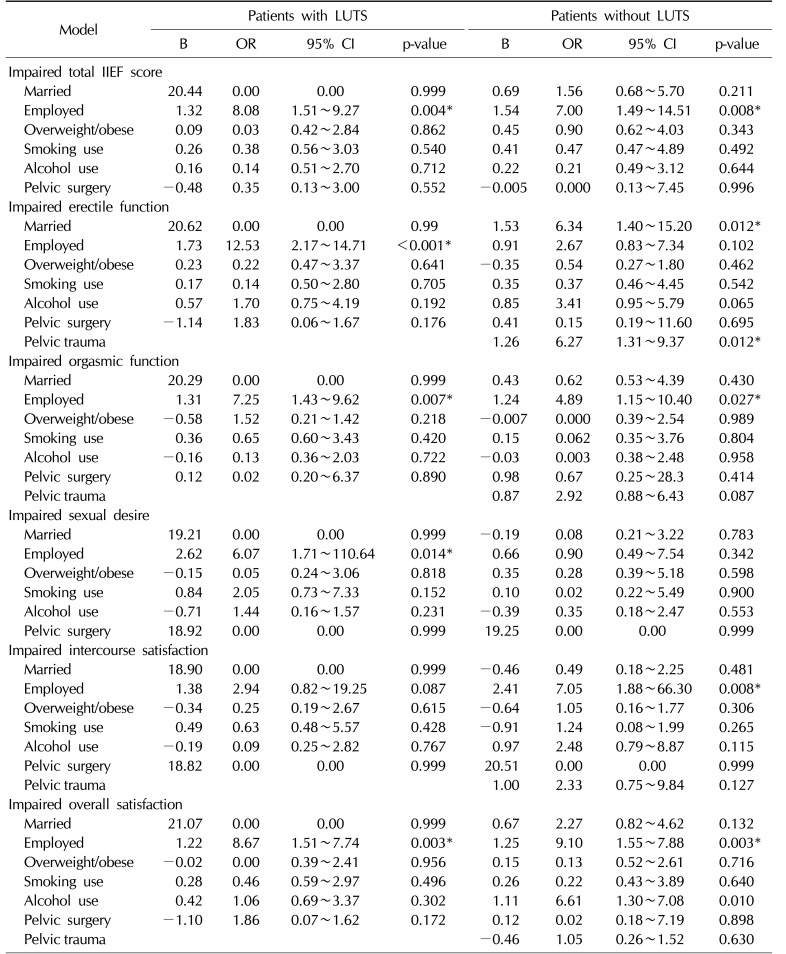
Table 4 shows the multivariate binary logistic regression of predictors of ED in men with and without LUTS. It was revealed that in men with LUTS, being currently employed predicted impaired total IIEF, EF, OF, SD, and OS, with odds ratios (ORs) of 8.08, 12.53, 7.25, 6.07, and 8.67, respectively (p<0.05).
In addition, in men without LUTS, currently being employed predicted impaired total IIEF, OF, IS, and OS, with ORs of 7.00, 4.89, 7.05, and 9.10, respectively (p<0.05). Moreover, Table 4 shows that in men without LUTS, being married only predicted impaired EF, with an OR of 6.34 (95% confidence interval [CI], 1.40∼15.20; p<0.05).
Finally, in men without LUTS, a positive history of pelvic trauma only predicted impaired EF, with an OR of 6.27 (95% CI, 1.31∼9.37; p<0.05).
We sought to determine the prevalence and predictors of ED among men with and without LUTS. It was found in this study that men with LUTS had a lower prevalence of ED than men without LUTS (64.6% and 73.7%, respectively). The lower prevalence in men with LUTS is in contrast to the results from the Cologne Male Survey of 8,000 men in Germany that showed the prevalence of ED to be 72% in men with LUTS, as compared to 38% in men without LUTS [10]. This difference may be connected with the fact that the Cologne study was better powered or with the possibility that more men without LUTS in Germany tended to avail themselves of treatment early enough to correct their symptoms. This hypothesis needs further evaluation.
Moreover, the high prevalence of 64.6% in this study suggests that a large proportion of men with LUTS in this country have ED but do not seek medical advice. This is in agreement with the results of the Multinational Survey of the Aging Male, which was conducted among approximately 14,000 men in the United States and six European countries. In that study, it was found that ED was strongly associated with LUTS [11].
Furthermore, the high prevalence of ED in both groups, with a similar mean age of 66 years, agrees with the results of the Massachusetts Male Aging Study, which showed that ED is a function of age. In that study, the prevalence of ED increased from 39% in men in their 40s to 67% in men in their 70s [17].
However, the high prevalence of ED in patients without LUTS shows that many patients are silently suffering from this disease. This is an indication that these men do not usually have enough confidence to seek medical advice. This is in agreement with the reports of Olarinoye et al [18], Olugbenga-Bello et al [19], and Ariba et al [20]. It is therefore imperative to develop means of helping patients build up the confidence to share their sexual problems with their physicians.
In our study, there was no significant difference in the mean total IIEF score (low effect size, Cohen d of 0.145; Table 2) between men with LUTS and men without LUTS. Power analysis revealed that in order for an effect of this size to be detected (with a 95% chance) as significant with a 5% level of error, a sample of 314 participants would be required. Hence, the likelihood of type 2 error was low in regard to the total IIEF score. However, in regard to the sub domains of IIEF, with smaller effect sizes (Cohen d as low as 0.054 for sexual desire), a sample size of 3,852 would be required to detect a significant difference with a power of 0.95.
Nonetheless, the mean values indicated poorer conditions in men with LUTS than in men without LUTS. This is in keeping with the fact that the burden of ED is greater in men with LUTS. Moreover, some researchers have argued that the pathophysiology underlying the association between LUTS and prostatic disease is still poorly understood [2122]. Nonetheless, the associated psychological burden of LUTS coupled with the disruption of social life and sleep disturbances could potentially lead to an inadequate sex life.
Furthermore, being employed was found to be a predictor of ED in men with LUTS. The OR for an impaired total IIEF score in these men was 8.08, with a 95% CI of 1.51∼9.27 (p<0.05). This may be connected with work fatigue or poor remuneration, which encourages engagement in other stressful activities outside of formal employment to improve incomes in this country. Both work fatigue and stressful engagements outside of formal employment could trigger psychogenic ED. Although this hypothesis requires further research to become absolutely reliable, it is in agreement with the fact that the psychological burden of LUTS predisposes men to ED [2122].
In addition, our study did not show any identifiable predictors of premature ejaculation (PE)/OS in men with LUTS. This is contrary to the findings of Lee [23] that ED and LUTS were significantly and independently correlated with PE/OS. However, Lee's work [23] focused on the severity of LUTS and ED. This difference may be because Lee's work was better powered or due to sociocultural differences between these two studies.
In contrast, the predictors of ED in men without LUTS identified in our study were being currently employed, being married, and having a previous history of pelvic trauma. While work fatigue may contribute to their psychological burden, as in men with LUTS, being married may make their sexual weaknesses easily noticeable, unlike those who are not married. Unmarried men may seek out sexual partners only when they feel adequately prepared. Moreover, the number of wives may be contributory to poor performance, although this was not adequately addressed by this study [24].
Pelvic trauma was found in this study to be a predictor of impaired erectile function in men without LUTS, with an OR of 6.27 (95% CI, 1.31∼9.37; p<0.05). This is in agreement with the report of Malavaud et al [25], in which ED was detected in 5% to 24% of pelvic fracture patients without a urethral injury. All our patients had stable pelvic fractures, which was also in line with the report of Malavaud et al [25]. This is in agreement with the fact that following pelvic fractures, neurogenic and vascular damage via direct or psychogenic mechanisms can occur [25].
Despite all the arguments for or against the differences in the prevalence of ED in men with and without LUTS, it should be noted that the preservation of sexual function is a vital component of quality of life for both groups of men, and should be considered sympathetically as part of the management of adult male patients in general. Both ED and LUTS affect sexual function, and hence quality of life [26].
The prevalence of ED was not found to be higher in men with LUTS than in men without LUTS. In men with LUTS, being employed was a predictor of ED. Being employed, being married, and having a positive history of pelvic trauma were predictors of ED in men without LUTS.
What are already known are as follows: 1) The global prevalence of ED has been on the increase, especially in patients with LUTS; 2) ED is underestimated in developing countries; 3) Early detection of ED is difficult, especially in developing countries.
What this study adds are as follows: 1) The prevalence of ED was high but underreported in men with and without LUTS in Nigeria; 2) A predictor of ED and impaired IIEF domain scores in men with LUTS was being currently employed; 3) Predictors of ED and impaired IIEF domains scores in men without LUTS were being currently employed, being married, and having a positive history of pelvic trauma; 4) The prevalence of ED in men without LUTS was higher than the prevalence of ED in men with LUTS in this study.
This study has some limitations. The sample size and hospital-based nature of this study make it difficult to extrapolate the results to the general population. Moreover, the inability of the IIEF-15 questionnaire to differentiate IIEF scores in men with more than one sexual partner is a serious drawback. This is because a man may have poor score for one partner but perform better with another partner. A community-based study is essential as a component of future research into this topic.
ACKNOWLEDGEMENTS
We wish to acknowledge the efforts of the urology staff nurses and the urology residents for their assistance in the management of these patients.
References
1. Shaeer KZ, Osegbe DN, Siddiqui SH, Razzaque A, Glasser DB, Jaguste V. Prevalence of erectile dysfunction and its correlates among men attending primary care clinics in three countries: Pakistan, Egypt, and Nigeria. Int J Impot Res. 2003; 15(Suppl 1):S8–S14. PMID: 12825103.

2. Eardley I. The incidence, prevalence, and natural history of erectile dysfunction. Sex Med Rev. 2013; 1:3–16. PMID: 27784558.

3. Idung AU, Abasiubong F, Ukott IA, Udoh SB, Unadike BC. Prevalence and risk factors of erectile dysfunction in Niger delta region, Nigeria. Afr Health Sci. 2012; 12:160–165. PMID: 23056022.

4. Altin M, Hughes RD, Williams R. Neutrophil adherence during hemoperfusion in fulminant hepatic failure. Int J Artif Organs. 1982; 5:315–317. PMID: 7174138.

5. Garko SB, Ogunsina MO, Danbauchi SS. Sexual dysfunction in hypertensive patients: implications for therapy. Ann Afr Med. 2005; 4:46–51.
6. Oyekanmi AK, Adelufosi AO, Abayomi O, Adebowale TO. Demographic and clinical correlates of sexual dysfunction among Nigerian male outpatients on conventional antipsychotic medications. BMC Res Notes. 2012; 5:267. PMID: 22676295.

7. Hackett GI, Milledge D. A 12-month follow up of 260 patients taking sildenafil. NHS clinical experience. In : Fourth Congress of the European Society for Sexual and Impotence Research (ESSIR); 2001 Sep 30∼Oct 3; Rome, Italy. poster 171.
8. Piores OM, Jimeno CA, Acampado LT. Erectile dysfunction among diabetes men at UP-PGH outpatient department: prevalence and risk factors. Phil J Internal Medicine. 2004; 42:197–202.
9. Oladiji F, Kayode OO, Parakoyi DB. Influence of sociodemographic characteristics on prevalence of erectile dysfunction in Nigeria. Int J Impot Res. 2013; 25:18–23. PMID: 22895099.

10. Braun M, Wassmer G, Klotz T, Reifenrath B, Mathers M, Engelmann U. Epidemiology of erectile dysfunction: results of the ‘Cologne Male Survey’. Int J Impot Res. 2000; 12:305–311. PMID: 11416833.

11. Rosen R, Altwein J, Boyle P, Kirby RS, Lukacs B, Meuleman E, et al. Lower urinary tract symptoms and male sexual dysfunction: the multinational survey of the aging male (MSAM-7). Eur Urol. 2003; 44:637–649. PMID: 14644114.

12. Shabsigh R, Anastasiadis AG. Erectile dysfunction. Annu Rev Med. 2003; 54:153–168. PMID: 12525671.

13. Montorsi F, Briganti A, Salonia A, Rigatti P, Burnett AL. Current and future strategies for preventing and managing erectile dysfunction following radical prostatectomy. Eur Urol. 2004; 45:123–133. PMID: 14733995.

14. Finger WW, Lund M, Slagle MA. Medications that may contribute to sexual disorders. A guide to assessment and treatment in family practice. J Fam Pract. 1997; 44:33–43. PMID: 9010369.
15. Braun MH, Sommer F, Haupt G, Mathers MJ, Reifenrath B, Engelmann UH. Lower urinary tract symptoms and erectile dysfunction: co-morbidity or typical “Aging Male” symptoms? Results of the “Cologne Male Survey”. Eur Urol. 2003; 44:588–594. PMID: 14572759.

16. Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997; 49:822–830. PMID: 9187685.

17. Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994; 151:54–61. PMID: 8254833.

18. Olarinoye JK, Kuranga SA, Katibi IA, Adediran OS, Jimoh AA, Sanya EO. Prevalence and determinants of erectile dysfunction among people with type 2 diabetes in Ilorin, Nigeria. Niger Postgrad Med J. 2006; 13:291–296. PMID: 17203117.
19. Olugbenga-Bello AI, Adeoye OA, Adeomi AA, Olajide AO. Prevalence of erectile dysfunction (ED) and its risk factors among adult men in a Nigerian community. Niger Postgrad Med J. 2013; 20:130–135. PMID: 23959355.
20. Ariba AJ, Oladopo OT, Iyaniwura CA, Dada OA. Management of erectile dysfunction: perceptions and practices of Nigerian primary care clinicians. SA Fam Pract. 2007; 49:16–29.

21. Shelbaia A, Elsaied WM, Elghamrawy H, Abdullah A, Salaheldin M. Effect of selective alpha-blocker tamsulosin on erectile function in patients with lower urinary tract symptoms due to benign prostatic hyperplasia. Urology. 2013; 82:130–135. PMID: 23711438.

22. Yong DJ, Lee KC, Cho IR. The effect of lower urinary tract symptoms on erectile function and the frequency of sexual intercourse. Korean J Urol. 2007; 48:458–462.

23. Lee JH. Associations between premature ejaculation, lower urinary tract symptoms, and erectile dysfunction in middle-aged Korean policemen. J Sex Med. 2014; 11:1512–1518. PMID: 24528521.

24. Gray P, Campbell B. Erectile dysfunction and its correlates among the Ariaal of northern Kenya. Int J Impot Res. 2005; 17:445–449. PMID: 16015378.

25. Malavaud B, Mouzin M, Tricoire JL, Gamé X, Rischmann P, Sarramon JP, et al. Evaluation of male sexual function after pelvic trauma by the International Index of Erectile Function. Urology. 2000; 55:842–846. PMID: 10840088.

26. Gacci M, Eardley I, Giuliano F, Hatzichristou D, Kaplan SA, Maggi M, et al. Critical analysis of the relationship between sexual dysfunctions and lower urinary tract symptoms due to benign prostatic hyperplasia. Eur Urol. 2011; 60:809–825. PMID: 21726934.

Table 1
Sociodemographic and clinical characteristics of the study population
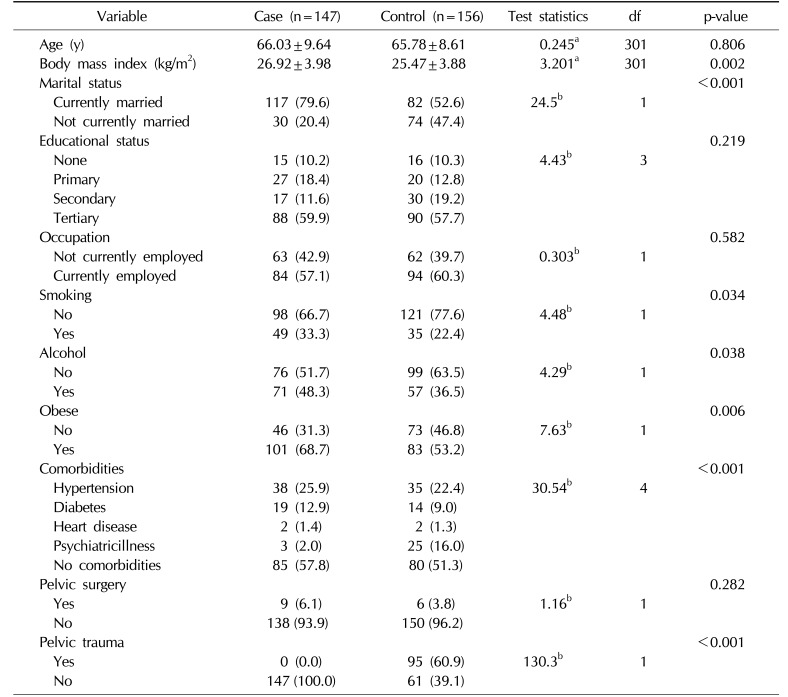
Table 2
Mean scores of the IIEF and its domains for the participants
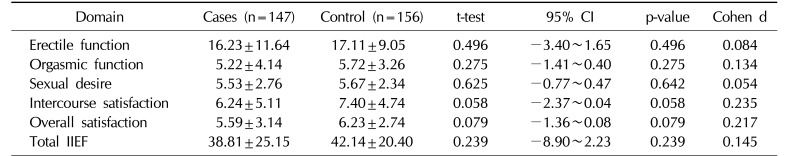
Table 3
Prevalence of participants with impaired IIEF scores
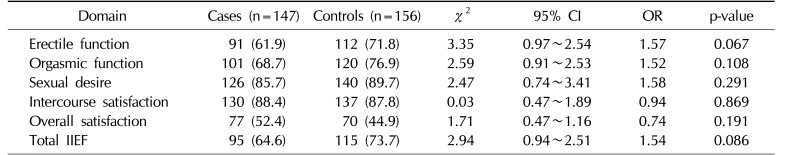
Table 4
Multivariate binary logistic regression of predictors of erectile dysfunction in patients with and without LUTS




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download